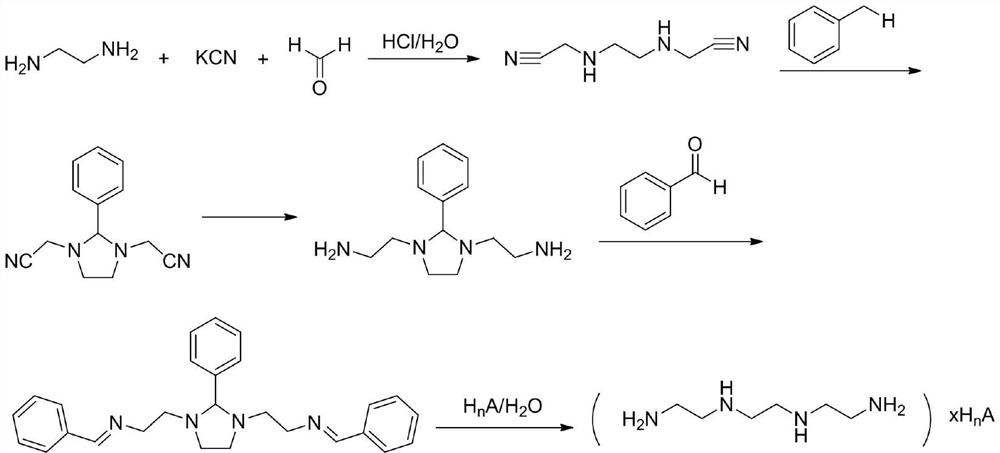
A kind of synthetic method of trientine hydrochloride
A synthesis method, hydrochloric acid technology, applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problems of poor molecular economy, expensive synthetic raw materials, unsuitable for industrial production, etc., and achieve short and safe synthesis routes Good sex, significant social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
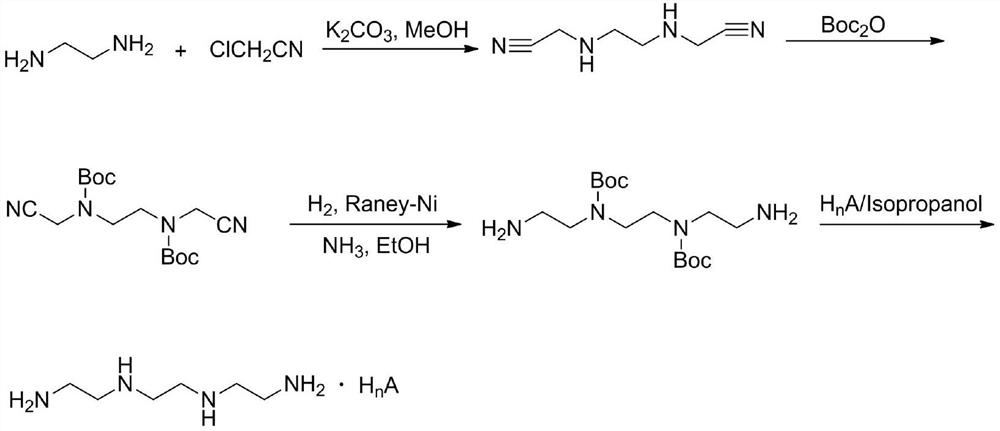
[0043] Step 1 Preparation of Intermediate I
[0044] The content of each component is: the molar ratio of ethylenediamine and di-tert-butyl dicarbonate is 2:1.
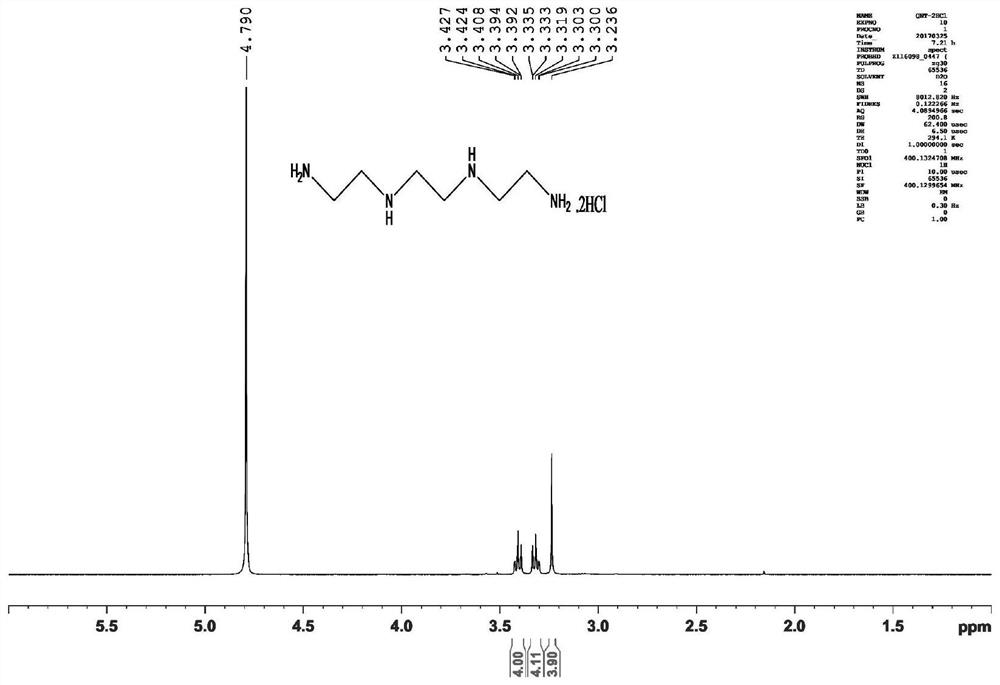
[0045] The preparation method is: in a 1L round bottom flask, add (2.7mL, 40mmol) ethylenediamine and 400mL chloroform, stir in an ice bath for 0.5h, then add (4.4g, 20mmol) Boc 2 O, the reaction solution was stirred at room temperature for 16 h and washed three times with 200 mL of saturated brine, the organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated to dryness under reduced pressure to obtain a colorless transparent liquid (intermediate I), with a yield of 98%. 1 H-NMR (400MHz, CDCl 3 )δ5.39(1H,s),3.05(2H,d,J=4.8Hz),2.64(2H,d,J=5.2Hz),1.29(9H,s),1.08(2H,s). 13 C-NMR (400MHz, CDCl 3 ) δ 156.2, 78.8, 43.3, 41.7, 28.3.
[0046] Step 2 Preparation of Intermediate II
[0047] The content of each component is: the molar ratio of intermediate I to 1,2-dibromoethane is 2:1.
[0048...
Embodiment 2
[0053] Step 1 Preparation of Intermediate I
[0054] The content of each component is: the molar ratio of ethylenediamine and di-tert-butyl dicarbonate is 8:1.
[0055] The preparation method is: in a 1L round bottom flask, add (5.4mL, 80mmol) ethylenediamine and 400mL chloroform, stir in an ice bath for 0.3h, then add (2.2g, 10mmol) Boc 2 O, the reaction solution was stirred at room temperature for 13 h, washed three times with 200 mL of saturated brine, the organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain a colorless transparent liquid (Intermediate I), with a yield of 49%.
[0056] Step 2 Preparation of Intermediate II
[0057] The content of each component is: the molar ratio of intermediate I to 1,2-dibromoethane is 4:1.
[0058] The preparation method is: in a 50mL round bottom flask, add 0.5g (3.12mmol) intermediate I, 0.15g (0.78mmol) 1,2-dibromoethane and 1.72g (12.48mmol) potassium carbonate, the...
Embodiment 3
[0063] Step 1 Preparation of Intermediate I
[0064] The content of each component is: the molar ratio of ethylenediamine and di-tert-butyl dicarbonate is 1:1.
[0065] The preparation method is: in a 1L round bottom flask, add (5.4mL, 80mmol) ethylenediamine and 400mL chloroform, stir in an ice bath for 0.8h, then add (17.4g, 80mmol) Boc 2 O, the reaction solution was stirred at room temperature for 20 h and washed three times with 200 mL of saturated brine, the organic phase was dried over anhydrous sodium sulfate, and the solvent was evaporated to dryness under reduced pressure to obtain a colorless transparent liquid (Intermediate I), with a yield of 63%.
[0066] Step 2 Preparation of Intermediate II
[0067] The content of each component is: the molar ratio of intermediate I to 1,2-dibromoethane is 4:1.
[0068] The preparation method is: in a 50mL round bottom flask, add 2g (12.5mmol) intermediate I, 0.59g (3.12mmol) 1,2-dibromoethane and 1.73g (12.48mmol) potassium c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com