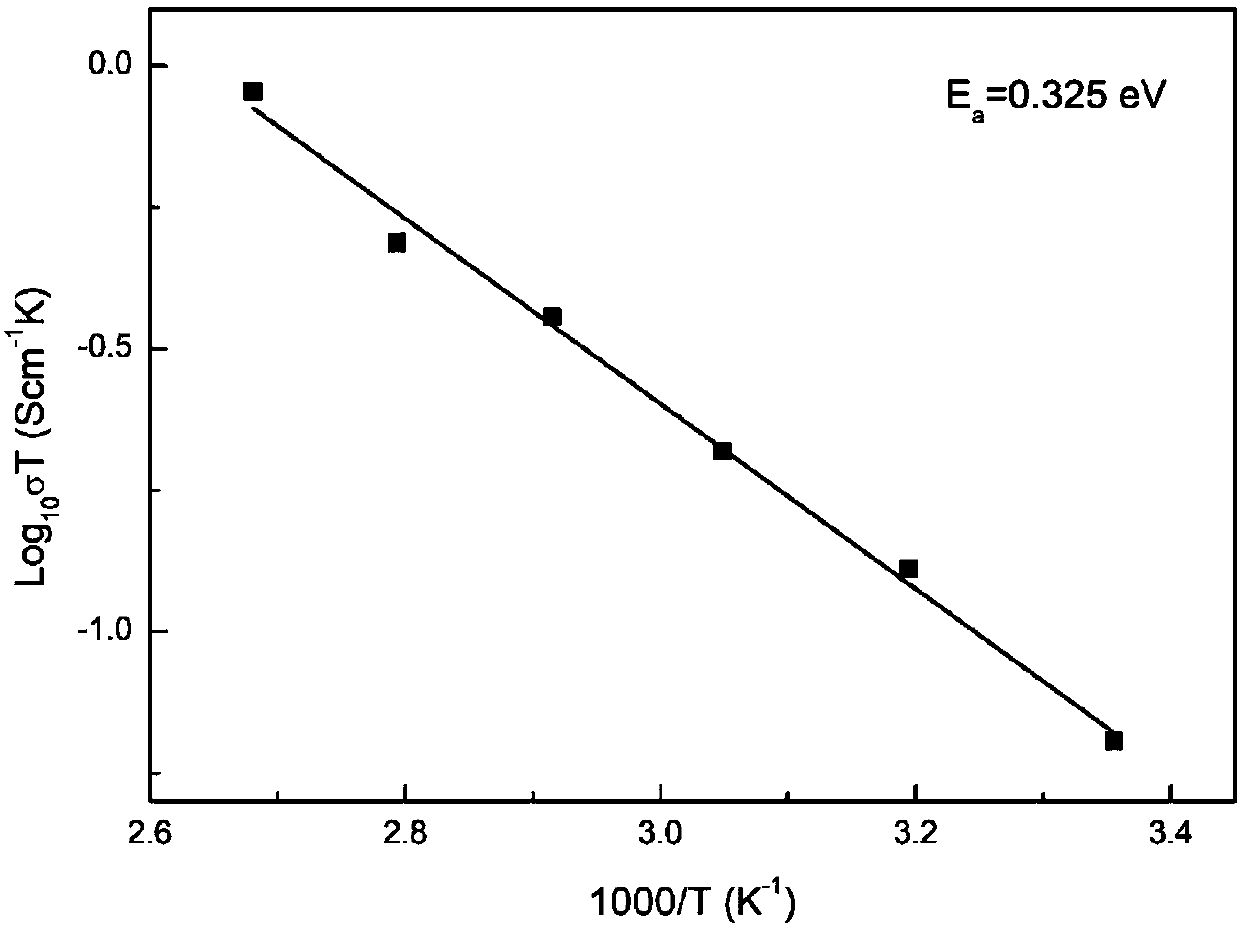
Divalent alkaline-earth metal and tantalum co-doped Li7La3Zr2O12 solid electrolyte material and preparation method
A technology of solid electrolyte and divalent alkali, which is applied in the field of new materials and electrochemistry, can solve problems such as low conductivity, greenhouse effect, and difficulty in densification, so as to improve conductivity, promote phase formation and sintering densification, and reduce migration and activation Can effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0026] The embodiment of the present invention provides a divalent alkaline earth metal and tantalum co-doped Li 7 La 3 Zr 2 o 12 Solid electrolyte material, the specific implementation method is as follows:
[0027] (1) Raw material mixing
[0028] The lithium source compound, the lanthanum source compound, the divalent alkaline earth metal compound, the zirconium source compound and the tantalum source compound are uniformly mixed according to the molar ratio of each element being 7-y+x:3-x:x:2-y:y. Wherein, 0<y<2, 0<x<y, in order to make up for the volatilization loss of the lithium source during the sintering process, the lithium source is in excess of 5% to 20%, preferably 10%. The mixing method is ball milling, and the ball milling medium can be organic solvents such as ethanol and isopropanol, among which isopropanol is preferred, and the spheres can be agate balls, zirconium balls, etc., among which zirconium balls are preferred. After ball milling, it was taken o...
Embodiment 1
[0036] Embodiment 1: prepare Li 6.5 La 2.9 Sr 0.1 Zr 1.4 Ta 0.6 o 12 Co-doped solid electrolyte materials
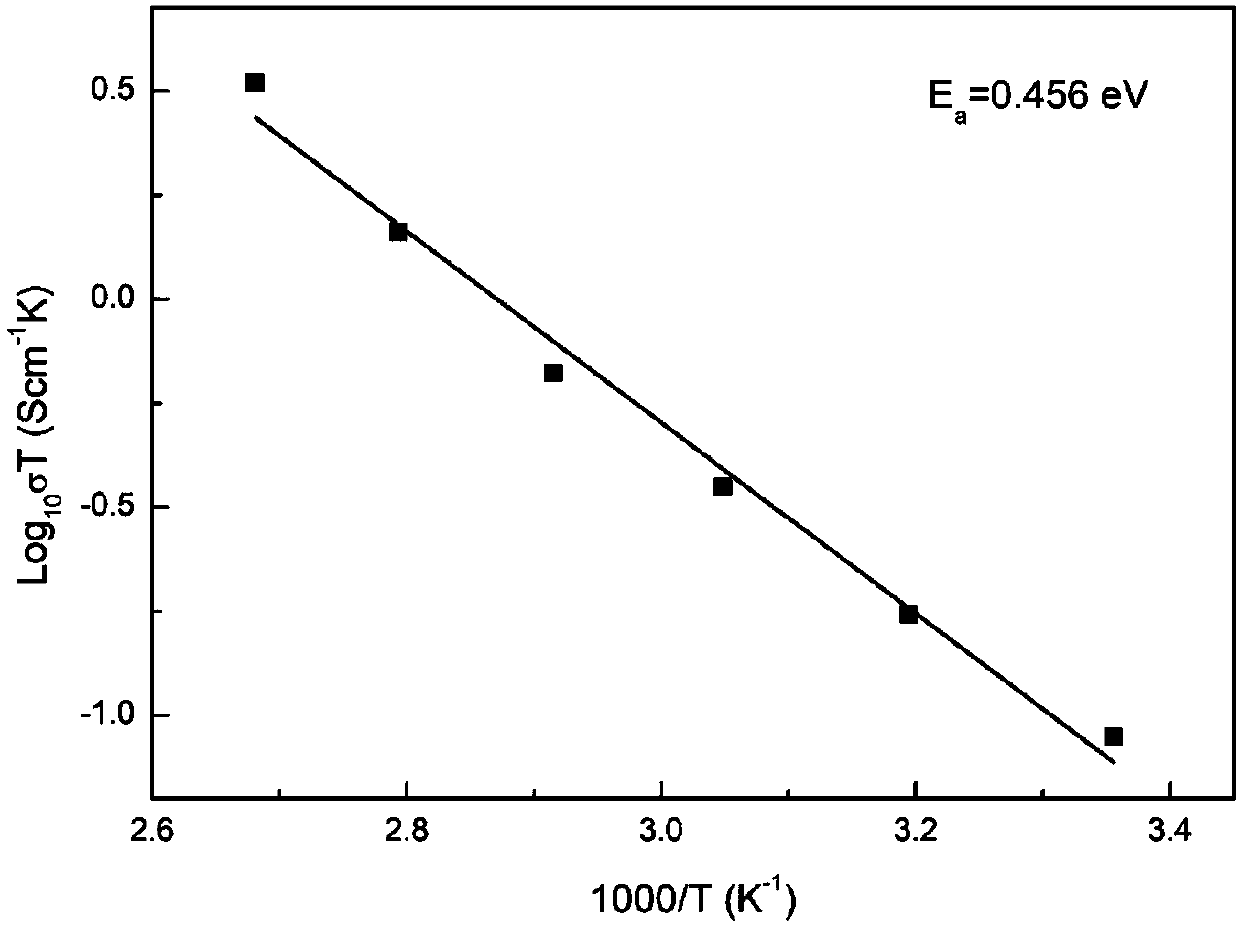
[0037] Weigh 1.321 grams of lithium carbonate, 2.362 grams of lanthanum oxide, 0.074 grams of strontium carbonate, 0.863 grams of zirconium oxide, and 0.663 grams of tantalum oxide according to the molar ratio of Li, La, Sr, Zr, and Ta of 6.5:2.9:0.1:1.4:0.6 , wherein the excess of lithium carbonate is 10%. Then ball mill in isopropanol medium for 12 hours, the speed of ball milling is 300 rpm, and dry treatment is carried out after the end. After heat treatment at 850°C for 12h, it was compressed into a tablet under a pressure of 300MPa, and then treated at 1100°C for 3h to obtain the Li 6.5 La 2.9 Sr 0.1 Zr 1.4 Ta 0.6 o 12 Co-doped solid electrolyte materials. The material has a lithium ion migration activation energy of 0.456eV, a total resistance of 139.8Ω at 25°C, a radius of 0.6175cm, a thickness of 0.05cm, and a conductivity of 2.99×10 -4 S / cm, the t...
Embodiment 2
[0038] Embodiment 2: prepare Li 6.6 La 2.6 Sr 0.4 Zr 1.2 Ta 0.8 o 12 Co-doped solid electrolyte materials
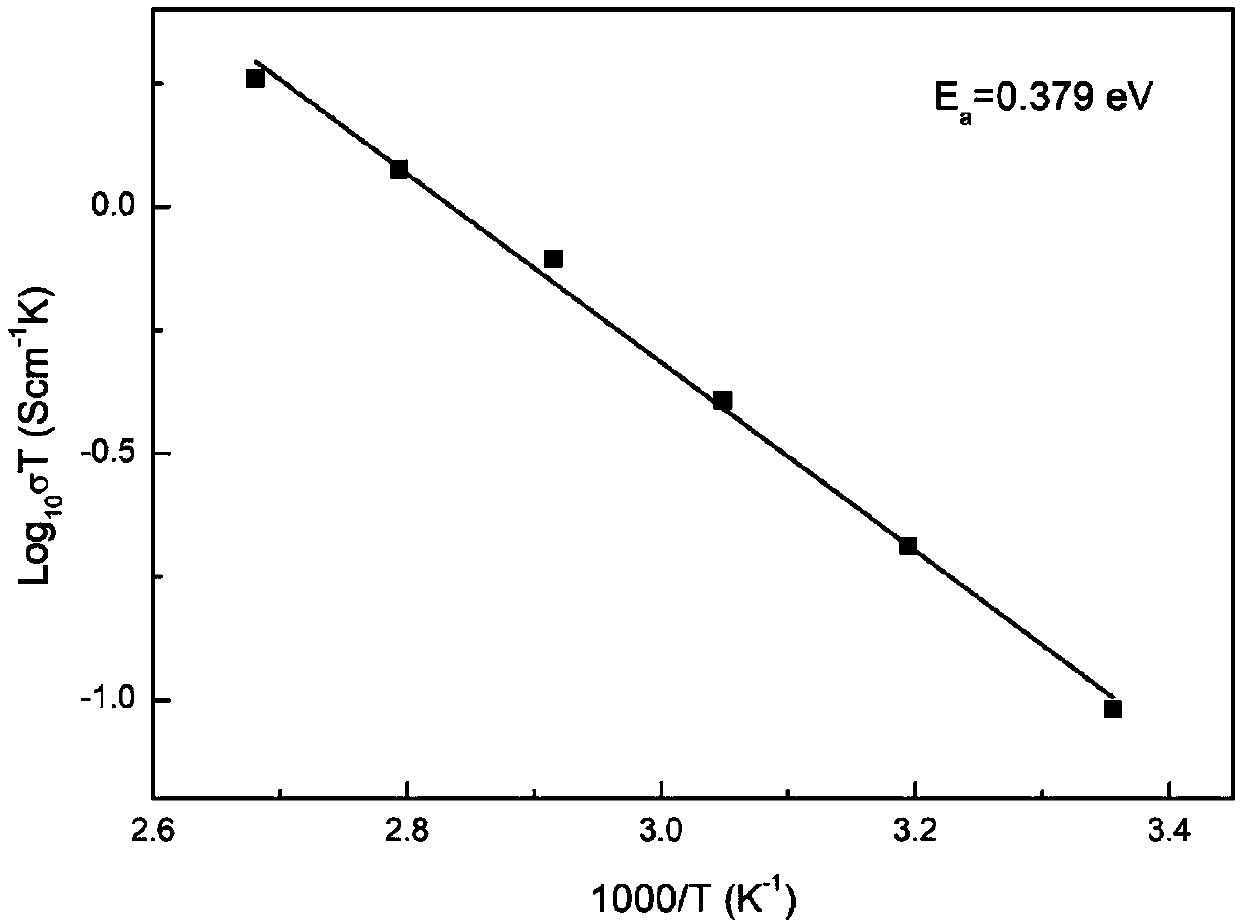
[0039] Weigh 1.341 grams of lithium carbonate, 2.118 grams of lanthanum oxide, 0.295 grams of strontium carbonate, 0.739 grams of zirconium oxide, and 0.884 grams of tantalum oxide according to the molar ratio of Li, La, Sr, Zr, and Ta of 6.6:2.6:0.4:1.2:0.8 , wherein the excess of lithium carbonate is 10%. Then ball mill in isopropanol medium for 12 hours, the speed of ball milling is 300 rpm, and dry treatment is carried out after the end. After heat treatment at 850°C for 12h, it was compressed into a tablet under a pressure of 300MPa, and then treated at 1100°C for 3h to obtain the Li 6.6 La 2.6 Sr 0.4 Zr 1.2 Ta 0.8 o 12 Co-doped solid electrolyte materials. The material has a lithium ion migration activation energy of 0.379eV, a total resistance of 155.3Ω at 25°C, a radius of 0.6165cm, a thickness of 0.06cm, and a conductivity of 3.21×10 -4 S / cm, indic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Total resistance | aaaaa | aaaaa |
| Radius | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com