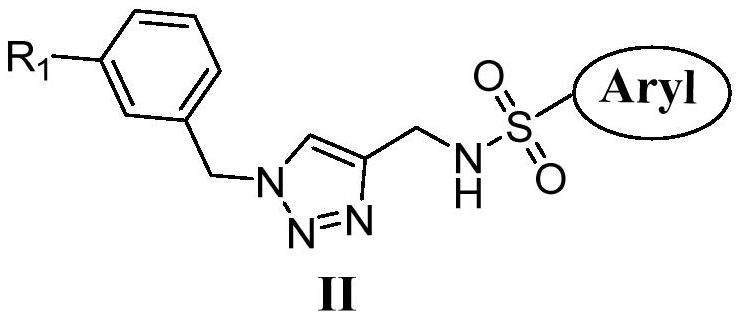
Sulfatriazole-type tubulin polymerization inhibitor and its synthesis method and application
A technology of triazole and sulfonamide is applied in the field of antitumor medicinal chemistry to achieve the effect of simple and efficient synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
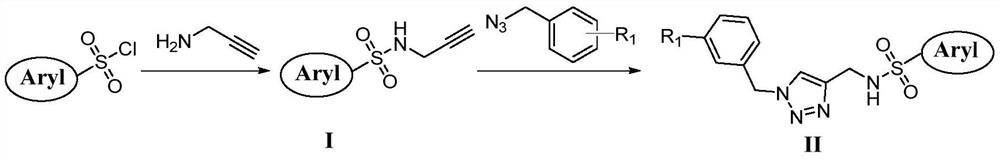
[0022] Example 1 Preparation of Compound (I)
[0023] The thiophene sulfonyl chloride (0.96 g, 5 mmol) and anhydrous potassium (1.38 g, 10 mmol) were mixed, and 10 ml of dichloroethane was added, alkyl amine (5 mmol) was added, and the temperature was increased to 60 ° C, and the reaction was continued. The TLC monitors the reaction process. After the reaction is completed, distilled water is added to the system, quench the reaction, then extract 3 times with dichloroethane, then use saturated brine to fetch dichloroethane phase 3 times, 10ml each time, finally The organic phase was dried over anhydrous magnesium sulfate, filtrates magnesium sulfate, and the filtrate was evaporated to remove dichloroethane. The obtained crude product was separated by silica gel column chromatography, petroleum ether / ethyl acetate = 10: 1 elution, obtained compound (I).
Embodiment 2
[0024] Example 2 Preparation of Formula (II)
[0025] Add compound (I) (5 mmol) and 3-methoxyphenylbenzyl glyzoid compound (6 mmol), then add 20 mlthf / h 2 O (10 ml / 10 ml) dissolved, and finally the copper sulfate (1 mmol) and sodium ascorbate (0.5 mmol) were added, and the mixture was stirred at room temperature overnight. The TLC monitors the reaction process. After the reaction is completed, distilled water was added to the system, then extracted 6 times with dichloroethane, and then withdraw the saline, the dichloroethane phase 3 times, 10ml each time, the final organic phase Magnesium sulfate dried, filtrates magnesium sulfate, and the filtrate was distilled off dichloroethane. The resulting crude product was separated by silica gel column chromatography, petroleum ether / ethyl acetate = 7: 1 elution, it was compound (IIA).
[0026] Compound IIB-IIL was prepared by compound IIA synthesis.
[0027] IIA: White solid, Yield: 80.1%, MP: 104-106 ° C. 1 H NMR (400 MHz, DMSO) δ8...
Embodiment 3
[0039] Example 3 Anti-tumor activity determination of the above compound:
[0040] The compounds used were synthesized and purified by the present invention; sample reserve: Weigh 3-5 mg sample placed in a 1.5 ml EP tube, then formulated with DMSO to form a concentration of 128 × 10 3 The solution of μg / ml, 4 ° C is stored, and the medium is diluted according to the desired concentration during the experiment. After taking the long-term cells, after the digestion, the cell density is tested with medium, inoculated into 96-well plates, 150 μl per well, after 24 h, cultured, cultured The group diluted drugs (50 μg / ml, 100 μg / mL), each concentration set 6 complex apertures, and a blank control group and a negative control group were provided. After the drug was 72h, 20 μl MtT was added to each well, and after 4 h, the liquid was subjected to 150 ml of DMSO, the oscillation, the enzyme detector 490 nm was detected, and the calculated inhibition rate, the calculation formula was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com