Paroxetine hydrochloride enteric-coated and sustained-release tablet and its preparation method
A technology of paroxetine hydrochloride and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as large individual differences, stability changes, and uncontrollability , to achieve significant controlled release characteristics, solve the problem of drug residues, and increase the effect of heat resistance and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
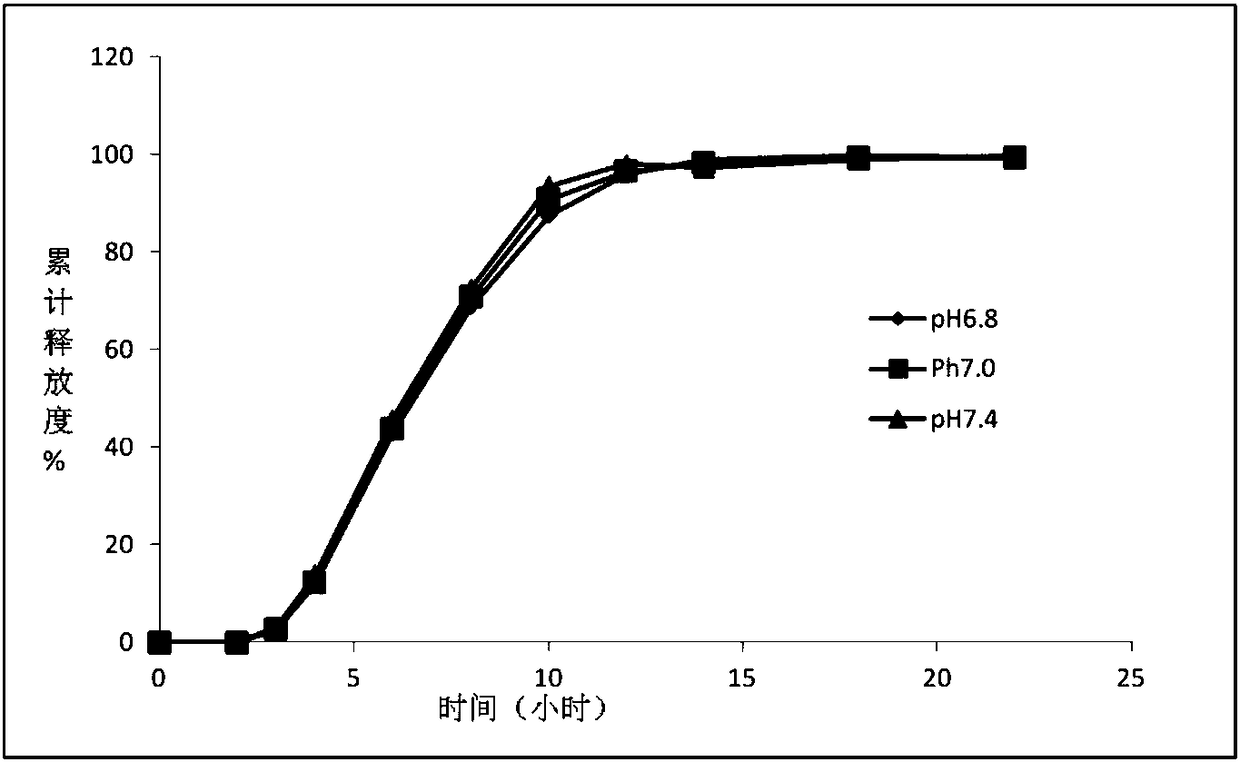
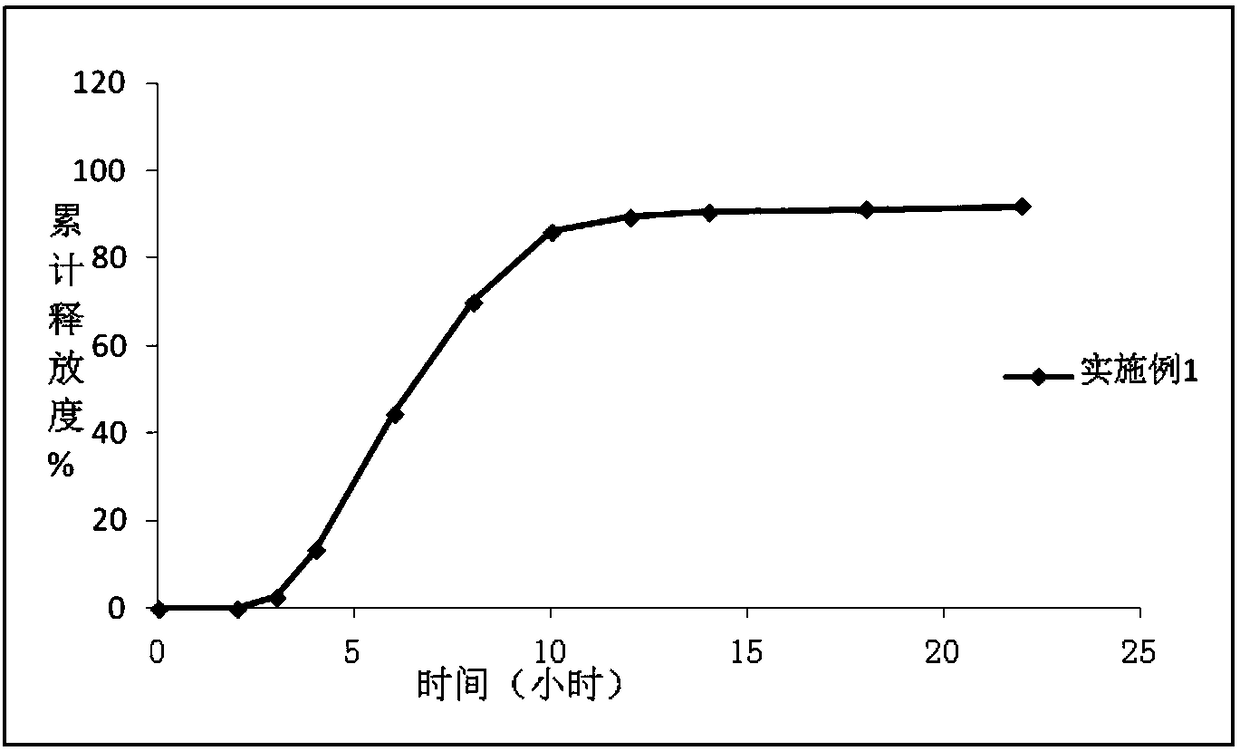
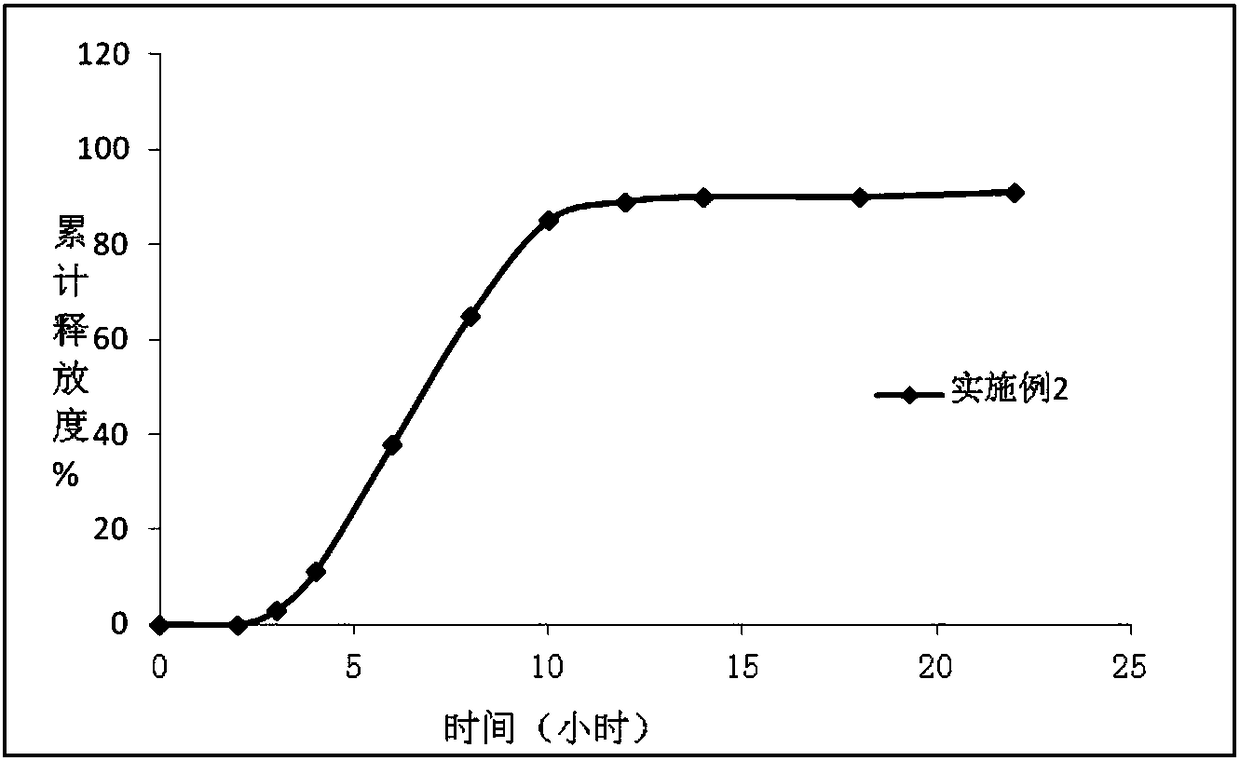
Image
Examples
Embodiment 1
[0034] A kind of paroxetine hydrochloride enteric-coated enteric-coated slow-release tablet, every 1000 tablets are prepared with the following raw materials and auxiliary materials:
[0035] Chip raw material:
[0036]
[0037] Raw material of isolation layer:
[0038] Hydroxyethylcellulose 6g
[0039] Polyethylene glycol 0.3g
[0040] Semi-permeable membrane material:
[0041] Cellulose acetate 10g
[0042] Macrogol 400 3.2g
[0043] Raw material of enteric coating:
[0044] L30D-55 8.5g
[0045] Triethyl citrate 0.25g
[0047] Moisture-proof clothing raw materials:
[0048] Opadry 13B150000 PINK 3.6g
[0049] The preparation method of the paroxetine hydrochloride enteric-coated enteric-coated sustained-release tablet comprises the following steps:
[0050] S1. Mix paroxetine hydrochloride with a particle size of 10 μm, lactose, microcrystalline cellulose 101, and povidone k30, add purified water to moisten, granulate through a 24-mesh ...
Embodiment 2
[0057] A kind of paroxetine hydrochloride enteric-coated enteric-coated slow-release tablet, every 1000 tablets are prepared with the following raw materials and auxiliary materials:
[0058] Chip raw material:
[0059]
[0060] Raw material of isolation layer:
[0061] Hydroxyethylcellulose 6g
[0062] Polyethylene glycol 3350 0.3g
[0063] Semi-permeable membrane material:
[0064] Cellulose acetate 9g
[0065] Macrogol 400 3g
[0066] Raw material of enteric coating:
[0067] L30D-55 8.5g
[0068] Triethyl citrate 0.25g
[0070] Film coating material:
[0071] Opadry 13B150000 PINK 3.6g
[0072] The preparation method of the paroxetine hydrochloride enteric-coated enteric-coated sustained-release tablet comprises the following steps:
[0073] S1. Mix paroxetine hydrochloride with a particle size of 15 μm, lactose, microcrystalline cellulose 101, and povidone k30, add purified water to moisten, granulate through a 40-mesh sieve, dry, ...
Embodiment 3
[0080] A kind of paroxetine hydrochloride enteric-coated enteric-coated slow-release tablet, every 1000 tablets are prepared with the following raw materials and auxiliary materials:
[0081] Chip raw material:
[0082]
[0083]
[0084] Raw material of isolation layer:
[0085] Hydroxyethylcellulose 6g
[0086] Polyethylene glycol 3350 0.3g
[0087] Semi-permeable membrane material:
[0088] Cellulose acetate 10g
[0089] Macrogol 400 3.2g
[0090] Raw material of enteric coating:
[0091] L30D-55 8.5g
[0092] Triethyl citrate 0.25g
[0094] Moisture-proof clothing raw materials:
[0095] Opadry 13B150000 PINK 3.6g
[0096] The preparation method of the paroxetine hydrochloride enteric-coated enteric-coated sustained-release tablet comprises the following steps:
[0097] S1. Mix paroxetine hydrochloride with a particle size of 50 μm, lactose, microcrystalline cellulose 101, and povidone k30, add purified water to moisten, granulate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com