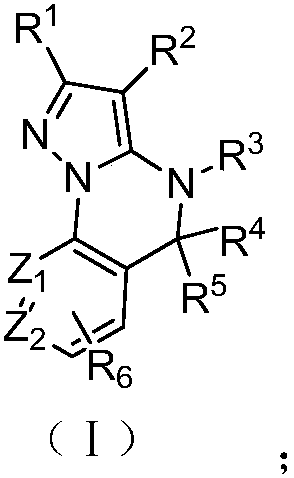
Fused heterocycle compounds containing pyrazolone rings and applications thereof
A technology of fused heterocyclic compounds, applied in the field of fused heterocyclic compounds, can solve the problems of restricting the development of agriculture and forestry, reducing the efficacy of pesticides, and restricting the application of pesticides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
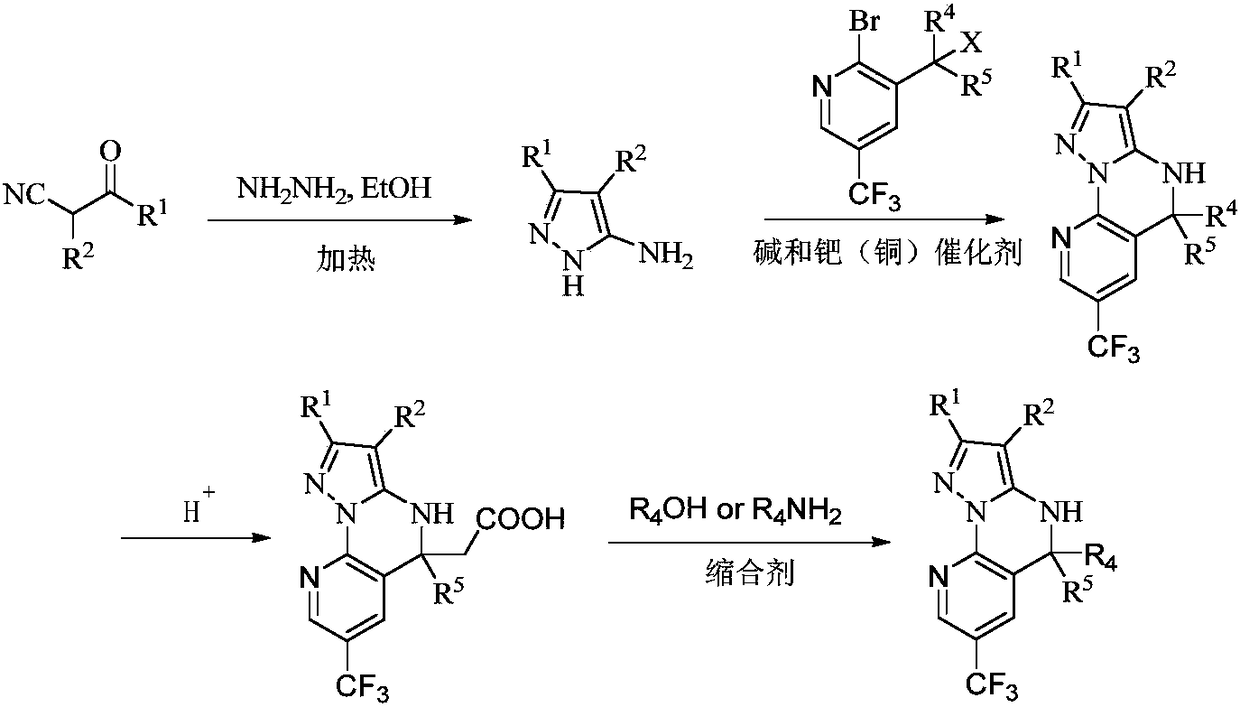
Embodiment 1
[0090] Embodiment 1 uses method A to synthesize a-1~a-138 compounds
[0091] Method A:
[0092]
[0093] Taking compound a-44 as an example, its specific preparation process is as follows:
[0094] S1: 5-Amino-1H-pyrazole-3-cyano
[0095] Add ethyl 2,3-dicyanopropionate (1.0 mmol) to 20.0 mL of ethanol, stir vigorously, add hydrazine hydrate (2.0 mmol) to the reaction solution, heat to reflux, stop heating after 3 hours, and cool to room temperature. The mixture was concentrated in vacuo, the mixture was extracted with ethyl acetate and water, and the extracted ethyl acetate was concentrated in vacuo to give 5-amino-1H-pyrazole-3-cyano as a white solid, which was used without further purification in in the next step.
[0096] S2: 5,5-dichloro-7-trifluoromethyl-4,5-dihydropyrazol[1,5-α]quinazoline[3,2-e]pyridine-2-cyano
[0097]Under the protection of nitrogen, 5-amino-1H-pyrazole-3-cyano (1.0mmol), 2-bromo-3-(trichloromethyl)-5-trifluoromethylpyridine (1.0mmol), iodide ...
Embodiment 2
[0105] Embodiment 2 uses method B to synthesize b-1~b-138 compound
[0106] Method B:
[0107]
[0108] Taking compound b-87 as an example, its specific synthesis process is as follows:
[0109] S1: 5-amino-1H-pyrazole-3-trifluoromethyl (reference Journal of medicinal chemistry (2017) 60:5099)
[0110] Under nitrogen protection, sodium hydride (2.5 mmol) was added to 20 ml of anhydrous tetrahydrofuran at 0°C, followed by anhydrous acetonitrile (2.0 mmol) and ethyl trifluoroacetate (1.0 mmol) were added to the reaction solution, heated to reflux, 20 After 2 hours, the heating was stopped, cooled to room temperature, and the mixture was concentrated in vacuo. The mixture was extracted with ether and water, the pH was adjusted to 2 by adding dilute hydrochloric acid, extracted with ether, and concentrated in vacuo to give 4,4,4-trifluoro-3-carbonylbutyronitrile as a brown oil, the intermediate was not further purified, used directly in the next step.
[0111] Add methanesu...
Embodiment 3
[0122] Example 3 Using method C to synthesize c-1~c-349 compounds
[0123] Method C:
[0124]
[0125] Taking compound c-27 as an example, its specific synthesis process is as follows:
[0126] S1: 6-Chloro-2-cyano-5-(2'2-diethoxy)-8-trifluoromethyl-4,5-dihydropyrazol[1,5-α]quinazoline- 5-ethyl carboxylate
[0127] Under nitrogen protection, 5-amino-1H-pyrazole-3-cyano (1.0mmol), 2-bromo-2-(2-bromo-6-chloro-4-trifluoromethylphenyl) malic acid diethyl A DMF mixture of ester (1.0 mmol), cuprous iodide (0.2 mmol) and cesium carbonate (0.5 mmol) was stirred at 100 °C for 24 hours. Cooled to room temperature, the mixture was filtered, concentrated in vacuo, and the residue was purified by column chromatography to obtain the target compound 6-chloro-2-cyano-5-(2'2-diethoxy)-8-trifluoromethyl-4, Ethyl 5-dihydropyrazolo[1,5-α]quinazoline-5-carboxylate (yield: 30%).
[0128] S2: 6-chloro-2-cyano-5-(2'2-diethoxy)-8-trifluoromethyl-3-trifluoromethylsulfinyl-4,5-dihydropyrazole[ ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com