Immunoenhancer and application thereof
An immune enhancer and an immune enhancement technology are applied in the field of swine foot-and-mouth disease vaccine compositions to achieve good immune effects, broad application prospects, and the effects of enhancing the level of cellular immune responses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
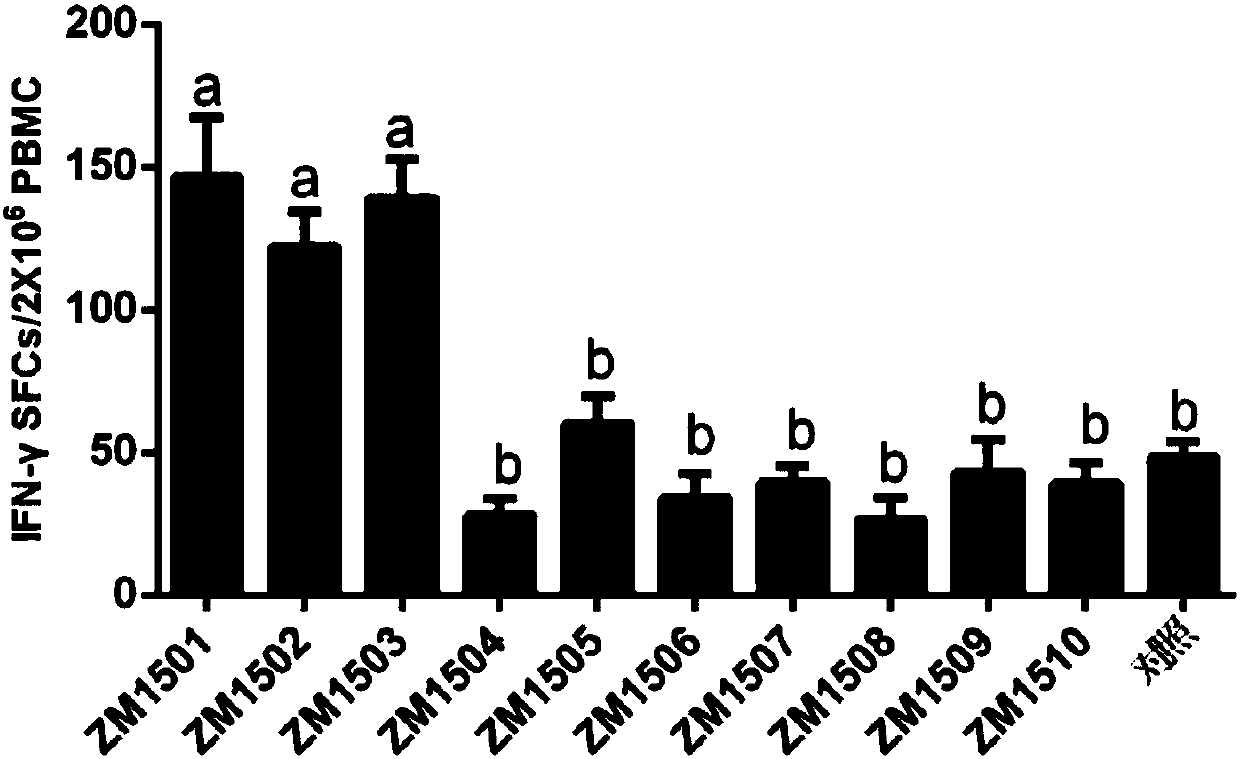
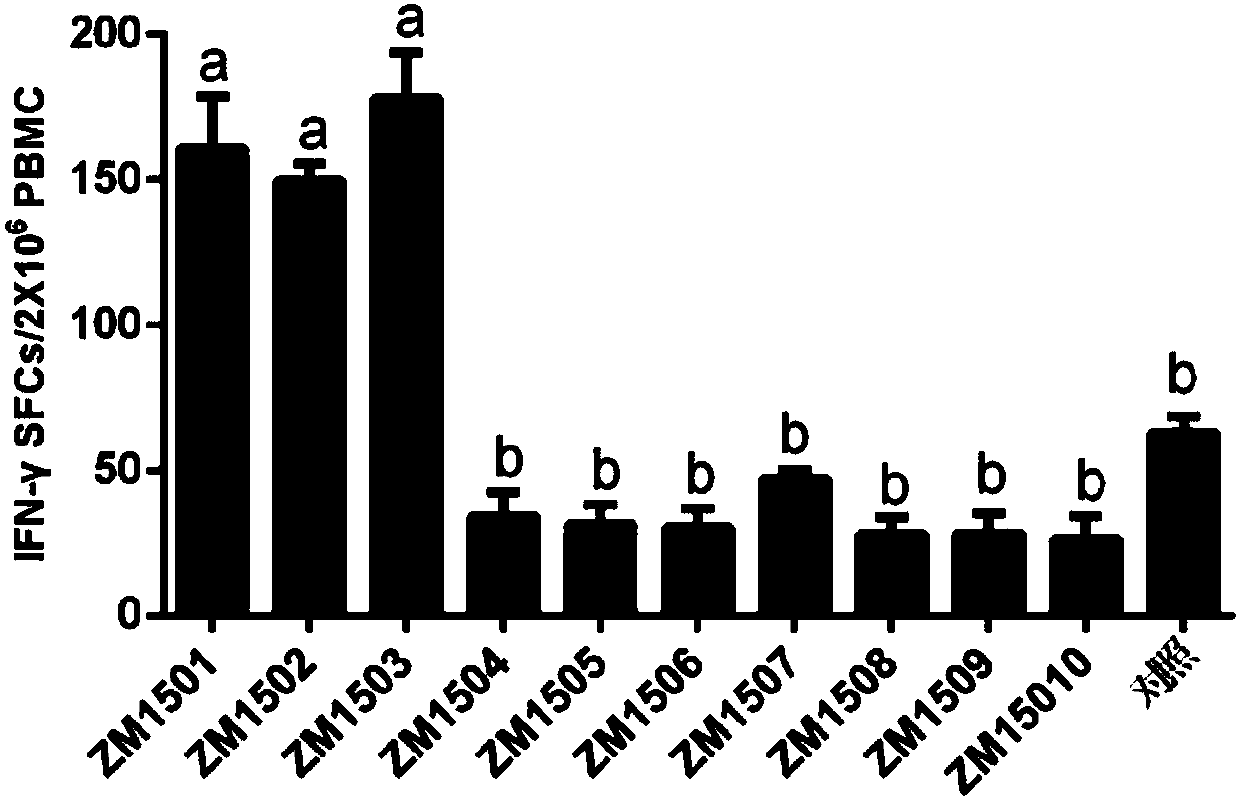
[0035] Example 1, Screening of CTL epitope polypeptides
[0036] It is predicted that after the polypeptide immunizes the body, the polypeptide antigen presented by MHC class I molecules can stimulate the body to produce memory T cells. Memory T cells induce the production of cytokines when the polypeptide antigen stimulates T cells again. Whether the predicted polypeptide is a CTL epitope polypeptide can be evaluated and analyzed through the detection of cytokine levels and biostatistical analysis.
[0037] 1. Prediction of peptides:
[0038] 10 predicted peptides ZM1501-ZM15010 were predicted and chemically synthesized from the website, and the sequence information is shown in the sequence list. 20mM Tris-HCl (pH 8.0) and 50mM NaCl buffer solutions were used to dissolve the predicted polypeptides respectively, and all 10 predicted polypeptides were prepared into a solution with a concentration of 1mg / ml. Each predicted polypeptide was mixed with 61 adjuvants at a volume...
Embodiment 2
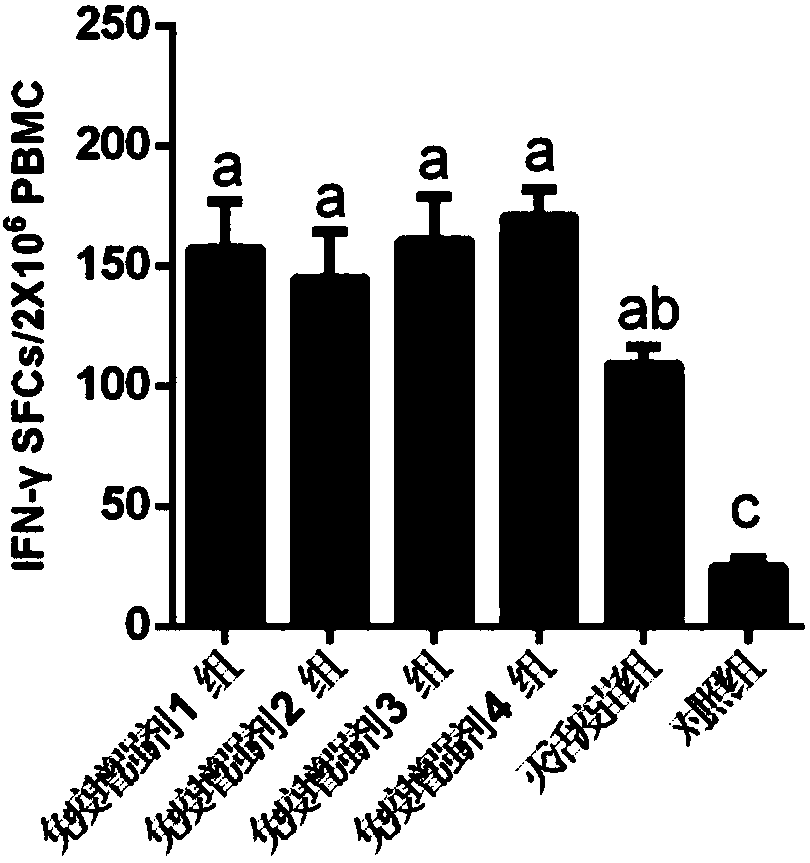
[0065] Example 2, Application of CTL Epitope-containing Polypeptide Vaccine Composition
[0066] 1. Three functional CTL epitope polypeptides obtained by screening in Example 1: ZM1501, ZM1502 and ZM1503 were dissolved in 20mM Tris-HCl (pH 8.0), 50mMNaCl buffer solution. The three CTL epitope polypeptides were prepared into a solution with a concentration of 1 mg / ml. in,
[0067] ZM1501 sequence: SADPVTATV (sequence 1 in the sequence listing)
[0068] ZM1501 sequence: ALDNTTNPT (sequence 2 in the sequence listing)
[0069] ZM1501 sequence: LM QTPSHTL (sequence 3 in the sequence listing)
[0070] According to the immunization dose and immunization group, take each CTL epitope polypeptide or prepare a mixed solution and mix it with the O / Mya98 / XJ / 2010 inactivated antigen containing the immunization dose, incubate overnight at 4°C, and mix the obtained mixed solution with Montanide ISA 206 adjuvant The porcine foot-and-mouth disease vaccine composition is prepared.
[0071...
PUM
| Property | Measurement | Unit |
|---|---|---|
| antibody titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com