A gene therapy drug for congenital black disease
A gene drug, gene technology, applied in gene therapy, drug combination, genetic engineering and other directions, can solve problems such as vision loss and rhodopsin content reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
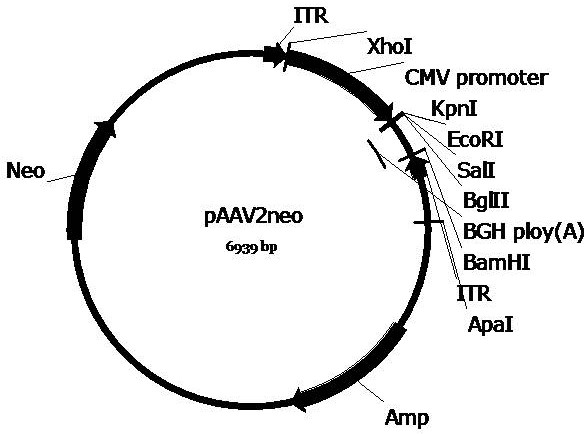
[0068] Example 1 Plasmid vector construction
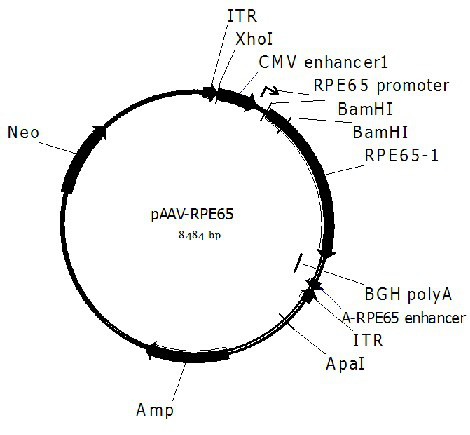
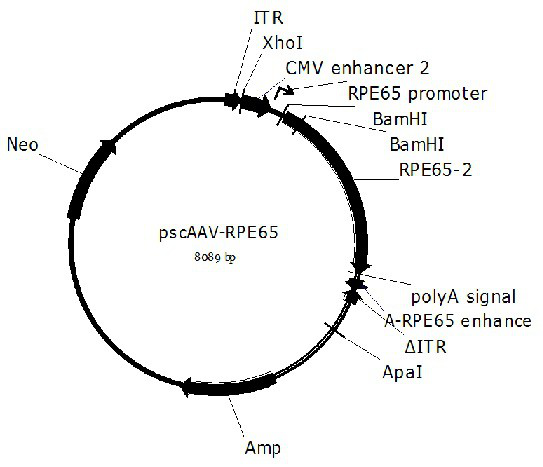
[0069] In order to construct the pscAAV-EGFP and pscAAV-RPE65 plasmids required for packaging the recombinant double-stranded AAV virus, we first constructed the pscAAV vector based on the pAAV2neo preserved by the company. Specifically, based on the left ITR sequence in the AAV2 genome (GenBankNo. AF043303), the trs sequence and the D sequence in the ITR sequence were deleted according to literature reports (Wang Z, et al . Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther . 2003; 10(26):2105-2111.), and obtained the ΔITR sequence (SEQ ID No.12). In order to facilitate the cloning operation, the sequence between 1392-1668bp in the pAAV2neo vector (the sequence between the ITR near the BGH polyA and the ApaI restriction site) was fused with the ΔITR sequence to obtain the fusion sequence. After adding BamHI and ApaI restriction sites at both ends of the fus...
Embodiment 2
[0083] Example 2 Preparation and assay of recombinant AAV virus
[0084] References (Xiao X, et al . Production of High-Titer Recombinant Adeno-Associated Virus Vectors in the Absence of Helper Adenovirus. J Virol . 1998; 72(3): 2224-2232.), using the three-plasmid packaging system to package and purify recombinant AAV virus. Briefly, the AAV vector plasmid (pAAV-EGFP, pAAV-RPE65, pscAAV-EGFP or pscAAV-RPE65), helper plasmid (pHelper) and AAV Rep and Cap protein expression plasmid (pAAV-R2C9) were prepared in a 1:1:1 ratio. After the molar ratio was mixed, HEK293 cells were transfected by the calcium phosphate method. After 48 hours of transfection, the cells and culture supernatant were harvested, and the recombinant AAV virus was isolated and purified by cesium chloride density gradient centrifugation. Four kinds of recombinant viruses such as ssAAV9-EGFP, ssAAV9-RPE65, scAAV9-EGFP or scAAV9-RPE65 were obtained by packaging and purification.
[0085] Quantitative PCR met...
Embodiment 3
[0090] Example 3 Expression of ssAAV9-EGFP and scAAV9-EGFP in mouse retinal pigment epithelial cell layer
[0091] Ten 8-week-old C57BL / 6J mice, half male and half male, were purchased from Beijing Huafukang Biotechnology Co., Ltd., randomly divided into two groups, and bred and raised in an SPF-level experimental animal center. The mice were reared in a 12-hour cycle of day and night, with a relative humidity of 60±10% and a temperature of 22±1ºC. The ssAAV9-EGFP and scAAV9-EGFP viruses were injected by the subretinal method. Each mouse was randomly selected to inject the left eye or the right eye, and the other eye was injected with an equal volume of sterile phosphate buffer as a control, and the injection dose of each eye was 1 μL with a concentration of 1×10 13 vg / ml virus solution. After 2 weeks, the mice were sacrificed, the retinas were separated, and frozen sections were used to observe the expression of green fluorescent protein in the RPE cells of the mice in each...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com