Methods, compositions and kits for extraction and purification of adeno-associated virus and adenovirus from host cells
An adenovirus and kit technology, applied in the fields of biotechnology and pharmaceutical research, can solve problems such as the existence of gradient medium health risks, cumbersome operating procedures, and reducing infection activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
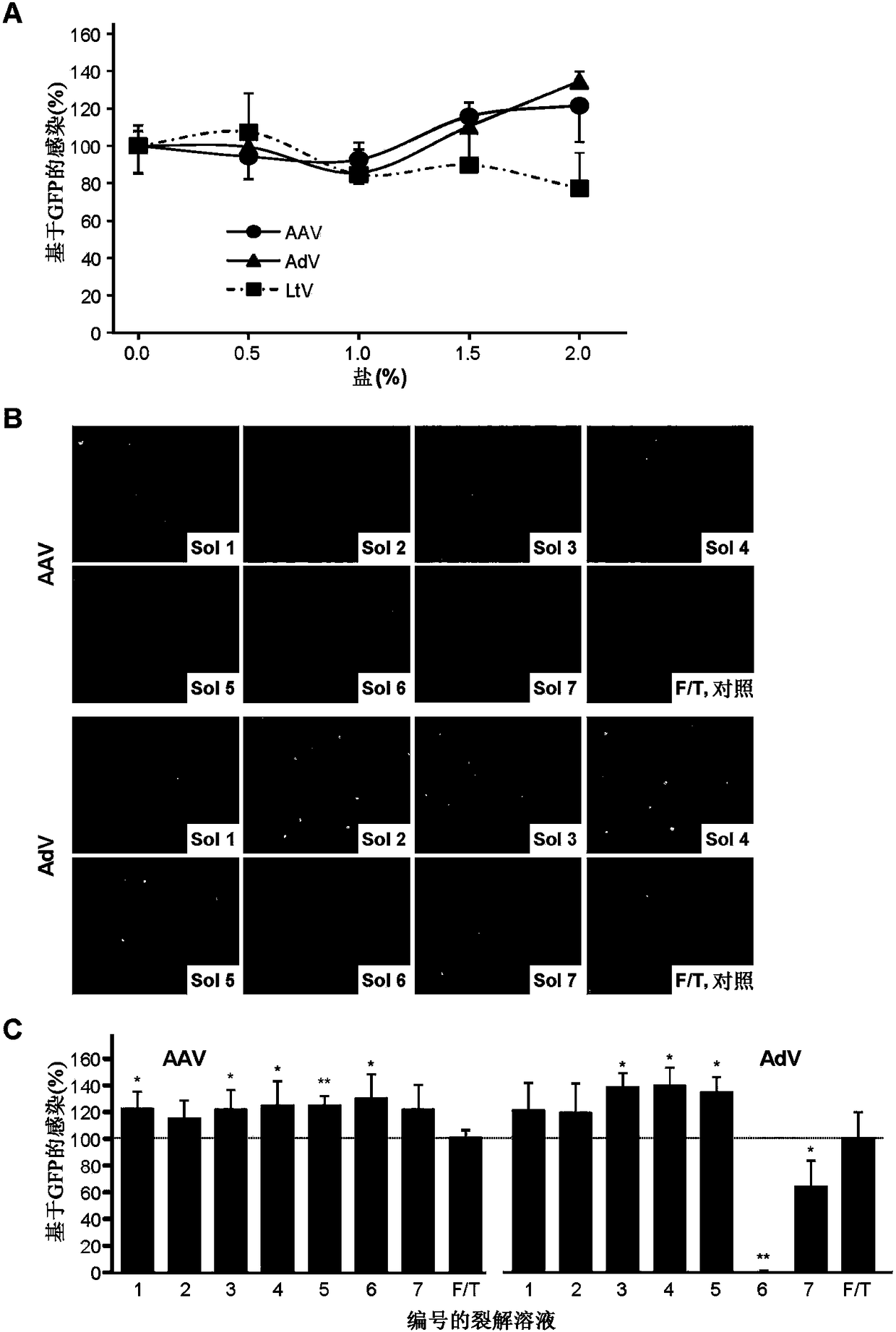
[0113] Example 1: Lysis, extraction and purification of adeno-associated virus and adenovirus from host cells
[0114] This example tests methods for the lysis, extraction and purification of AAV and AdV virus particles from their packaging cells (host cells). This example demonstrates that the present method achieves higher recovery and purity of virus particles compared to conventional methods. Furthermore, the method is easy to operate and can be scaled up readily.
[0115] Methods and Materials
[0116] Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and cell culture dishes were purchased from Fisher Scientific. 6-well and 12-well cell culture plates were purchased from Santa Cruz Biotechnology. Plasmid DNA Maxiprep kit was purchased from Qiagen. Lipofectamine 2000 Transduction Reagent (TransfectionReagent) was purchased from Life Technologies. DNase I, Maxima Sybr Green qPCR master mix (MasterMix, 2X), Pierce BCA protein assay kit, SDS-PAGE minige...
Embodiment 2
[0175] Embodiment 2: virus purification kit
[0176] This example tests a collection of reagents and materials, referred to as virus purification kits, for the purification of some virus particles. This example illustrates that the kit can greatly improve the quality and efficiency of virus purification.
[0177] Methods and Materials
[0178] Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and cell culture dishes were purchased from Fisher Scientific. 6-well and 12-well cell culture plates were purchased from Santa Cruz Biotechnology. Plasmid DNA Maxiprep kit was purchased from Qiagen. Lipofectamine 2000 transduction reagent was purchased from Life Technologies. DNase I, Maxima Sybr Green qPCR master mix (2X), Pierce BCA protein assay kit, SDS-PAGE mini gels, Amicon Ultra-4 centrifugal filters and all chemicals were purchased from FisherScientific. Silica-based chromatography columns were purchased from Agilent Technologies, Inc.
[0179] cell cult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com