Preparing method for carboxylation porous crosslinked polystyrene copolymerized fluorescent microspheres
A technology of cross-linked polystyrene and fluorescent microspheres, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of lack of functional groups on the surface of fluorescent microspheres, inconvenient biological research, leakage of fluorescent dyes, etc., and achieve the goal of preparing Low cost, uniform fluorescence intensity distribution, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Preparation of polystyrene seed balls
[0053] It is prepared by dispersion polymerization method: respectively take 0.024 g of initiator azobisisobutyronitrile, 0.072 g of dispersant polyvinylpyrrolidone, and 9.0 g of absolute ethanol in a micro-reactor, and ultrasound to obtain a clear and transparent solution. Add 1.2 g of styrene to the solution, purge nitrogen to remove oxygen, and seal. Place it in a constant temperature shaker and react for 12 hours at 70°C and 130 rpm. The obtained microspheres are washed repeatedly with water and ethanol successively, and dried under vacuum at 50°C to obtain polystyrene seed balls.
[0054] (2) Synthesis of allylcarbazole
[0055] Add 0.69g (4.00mmol) of carbazole, 0.62g (6.00mmol) of 3-bromopropene, 1.57g (28.00mmol) of KOH, hydroquinone and KI (trace amount) into a 100mL four-neck flask equipped with electromagnetic stirring. And dried DMF 10mL. In N 2 Under protection and dark conditions, the reaction mixture was heated to ...
Embodiment 2
[0068] (1) Preparation of polystyrene seed balls
[0069] It is prepared by dispersion polymerization method: respectively take 0.024 g of initiator azobisisobutyronitrile, 0.072 g of dispersant polyvinylpyrrolidone, and 9.0 g of absolute ethanol in a micro-reactor, and ultrasound to obtain a clear and transparent solution. Add 1.2 g of styrene to the solution, purge nitrogen to remove oxygen, and seal. Place it in a constant temperature shaker and react for 12 hours at 70°C and 130 rpm. The obtained microspheres are washed repeatedly with water and ethanol successively, and dried under vacuum at 50°C to obtain polystyrene seed balls.
[0070] (2) Synthesis of allyl fluorescein
[0071] Synthesized according to the literature (J.Mater.Chem.,2009,19,2018-2025) method:
[0072]
[0073] (3) Activation of bulb
[0074] Weigh 0.1g of polystyrene seed balls and disperse them in a 0.25% sodium lauryl sulfate solution with ultrasound to obtain a uniformly dispersed seed ball solution. At th...
Embodiment 3
[0084] (1) Preparation of polystyrene seed balls
[0085] It is prepared by dispersion polymerization method: respectively take 0.024 g of initiator azobisisobutyronitrile, 0.072 g of dispersant polyvinylpyrrolidone, and 9.0 g of absolute ethanol in a micro-reactor, and ultrasound to obtain a clear and transparent solution. Add 1.2 g of styrene to the solution, purge nitrogen to remove oxygen, and seal. Place it in a constant temperature shaker and react for 12 hours at 70°C and 130 rpm. The obtained microspheres are washed repeatedly with water and ethanol successively, and dried under vacuum at 50°C to obtain polystyrene seed balls.
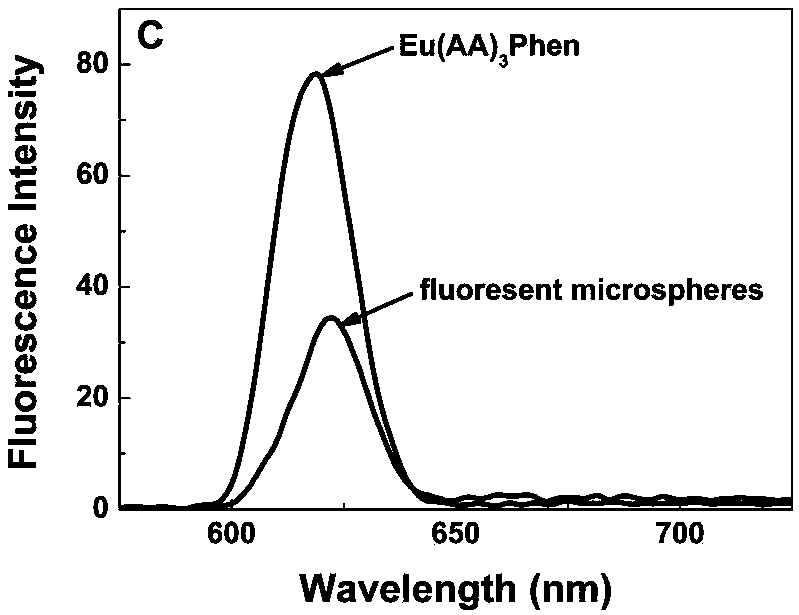
[0086] (2) Eu(AA) rare earth europium complex with double bond 3 Synthesis of Phen
[0087] Called Eu 2 O 3 (0.70g, 2mmol) add concentrated hydrochloric acid to dissolve and steam until nearly dry, add 40mL absolute ethanol to dissolve, make EuCl 3 Ethanol solution, the reaction formula is as follows:
[0088] Eu 2 O 3 +6HCl→2EuCl 3 +3H 2 O
[0089] W...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com