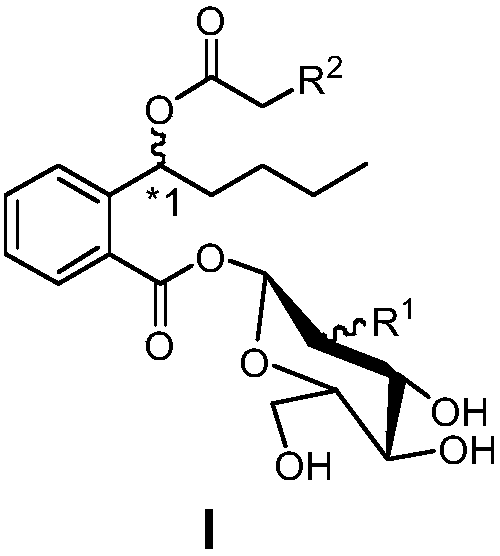
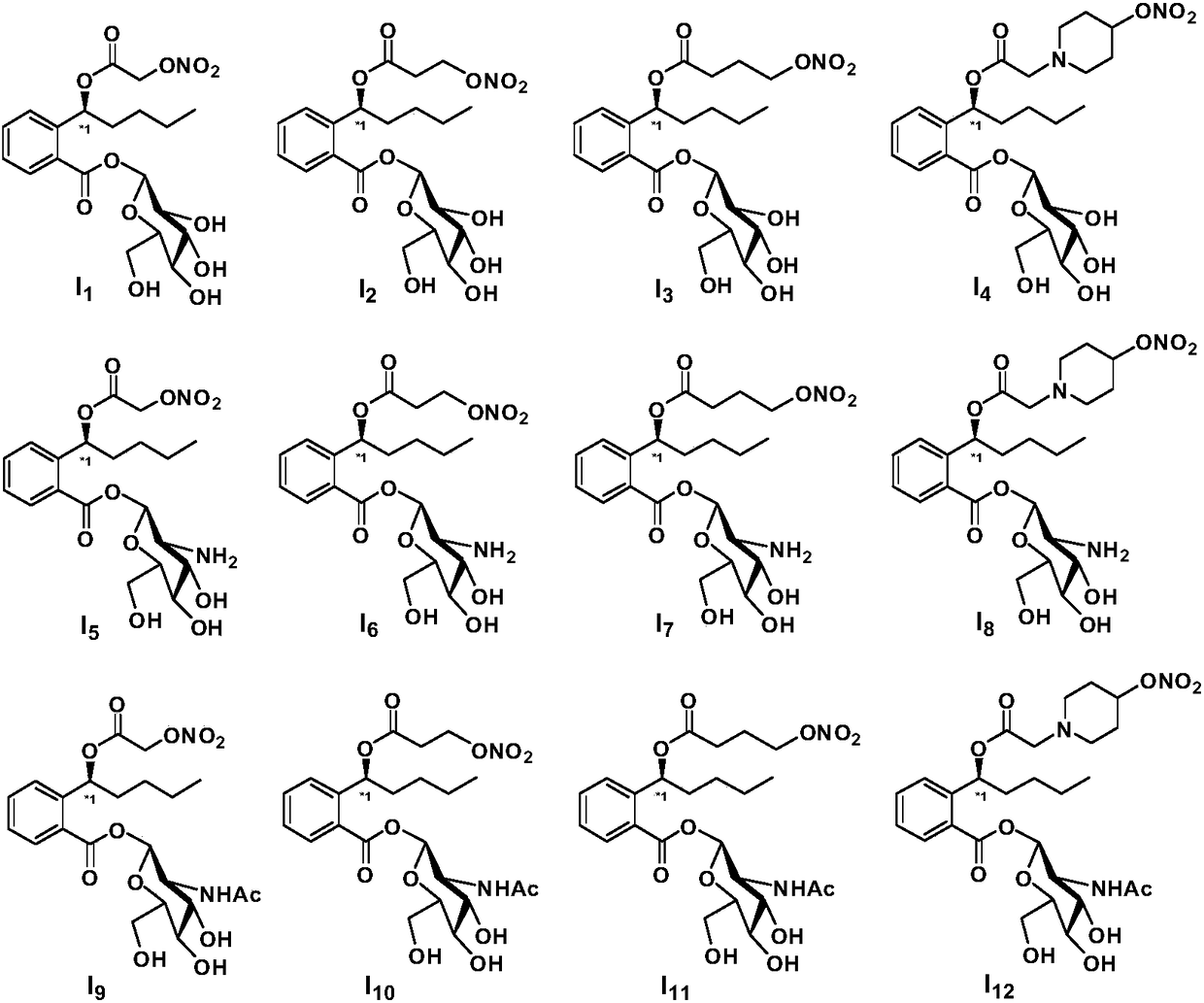
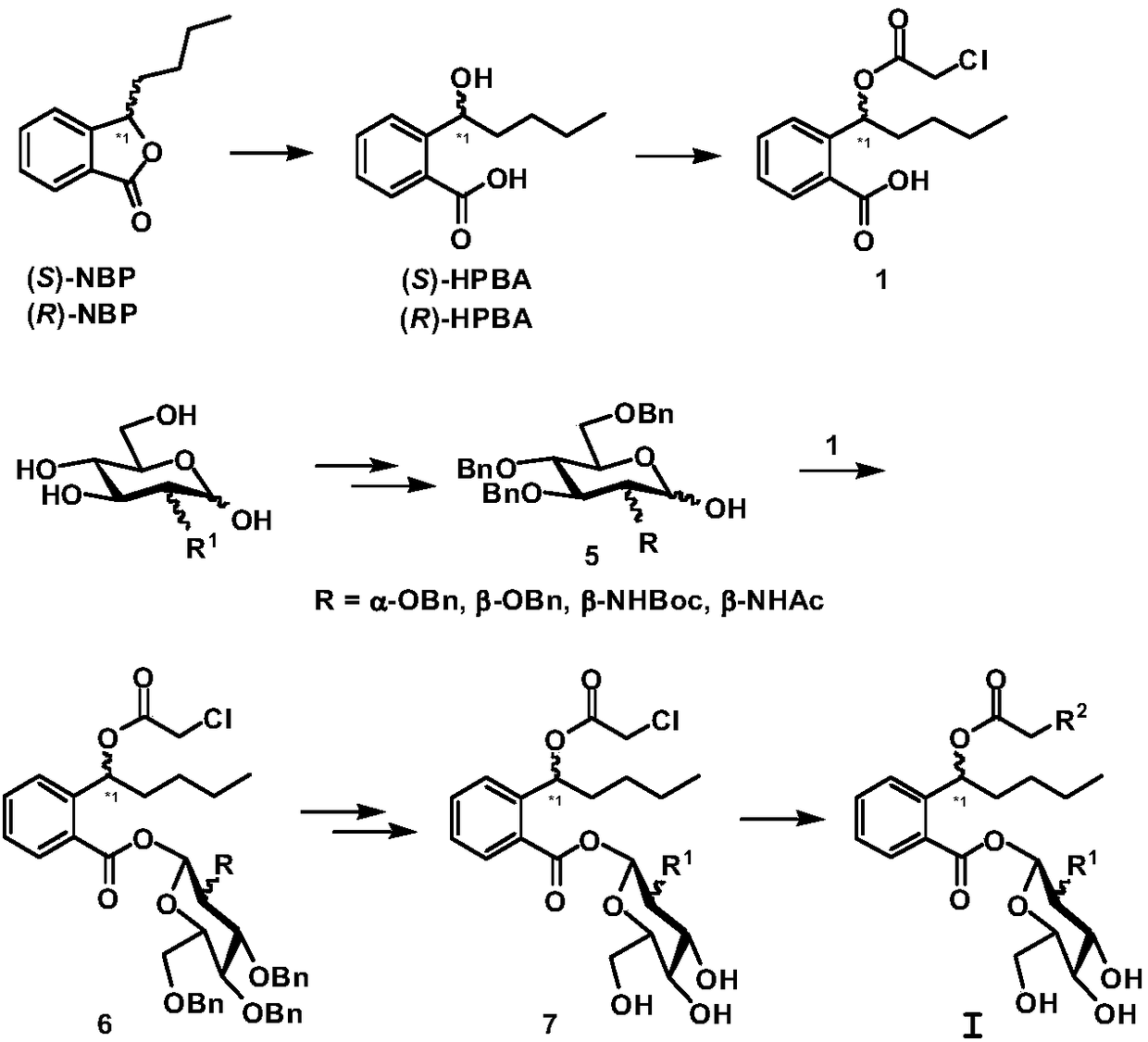
Preparation method and pharmaceutical applications of glycosyl modified butylphthalide ring-opened derivatives
A technology of glycosyl modification and butylphthalide, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of complex synthesis and poor stability of sugar-based compounds, and achieve the effect of low drug prices.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Compound I 1 preparation of
[0044] Compound 7 (2.0 mmol) was dissolved in 20 mL of anhydrous acetonitrile, silver nitrate (3.0 mmol) was added, and reacted at 60-70° C. for 2-6 h in the dark. After completion of the reaction, cool to room temperature, filter, concentrate under reduced pressure, dissolve the concentrate in ethyl acetate, stir at room temperature for 10 min, filter, concentrate under reduced pressure, flash column chromatography (PE / EtOAc=5 / 1–1 / 1, v / v) 851 mg of light yellow oil was obtained, the yield was 90%. ee=99.1%.MS(ESI):m / z 474.4[M+H] + . 1 H NMR (300Hz, CDCl 3 ):δ0.89(t,3H,J=6.8Hz),1.24–1.36(m,4H),1.52–1.64(m,2H),3.38–3.42(m,1H),3.45–3.50(m,1H ),3.62–3.68(m,2H),3.70–3.78(m,1H),4.00–4.06(m,1H),4.49(s,2H),6.18(s,1H),6.25–6.32(m,1H ),7.30–7.35(m,2H),7.42(m,1H),7.90(d,1H,J=7.8Hz). 13 C NMR (75MHz, CDCl 3 ):δ171.2,167.9,140.8,132.6,130.6,129.7,128.2,125.8,99.1,75.4,73.1,72.2,70.1,68.8,68.2,59.5,36.6,26.4,23.3,14.0.HRMS(ESI):m / z c...
Embodiment 2
[0045] Embodiment 2 pharmacological test
[0046] In vitro anti-platelet aggregation activity research, the experimental steps are as follows:
[0047] Experimental method: 10 male rabbits, weighing 2.0-2.5kg. The animals were fed for one week under the conditions of 25° C. and relative humidity of 60 to 75%, and then used for experiments. Take 2 rabbits, use lidocaine for local anesthesia, surgically separate the common carotid artery, take blood, take 3.8% sodium citrate 1:9 anticoagulation, and centrifuge at 500r / min for 10min to prepare platelet-rich plasma (PRP), and the remaining Part of it was centrifuged at 3000r / min to prepare platelet-poor plasma (PPP), and the platelet aggregation experiment was carried out by turbidimetric method. Add 240 μL of PRP and 30 μL of different concentrations of test drugs into the measurement tube, incubate for 5 min, add 30 μL of adenosine diphosphate (ADP) (final concentration of 10 μmol / L), 30 μL of thrombin (final concentration of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com