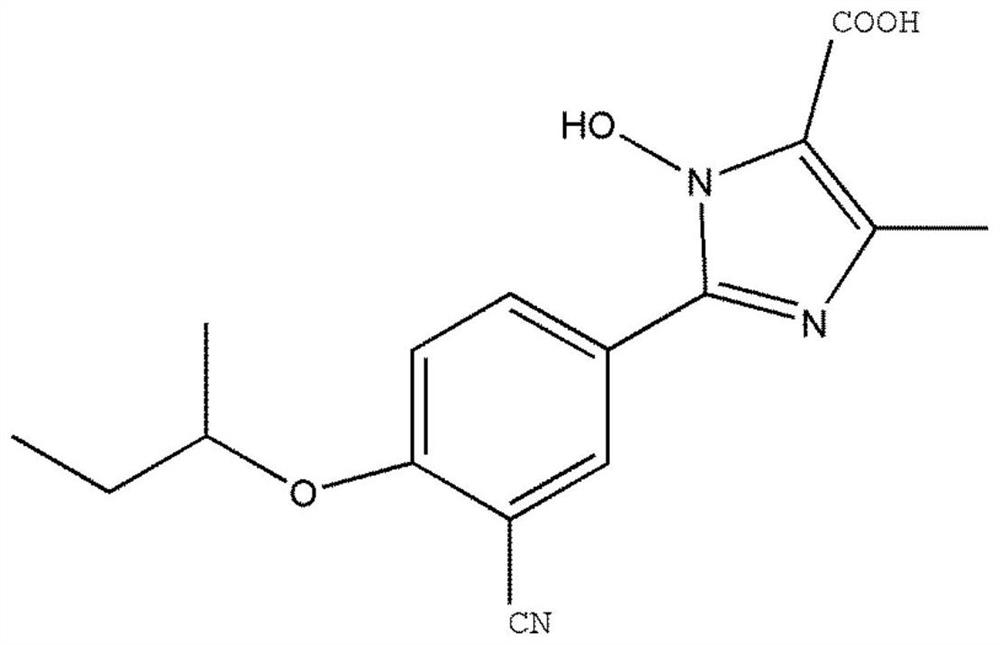
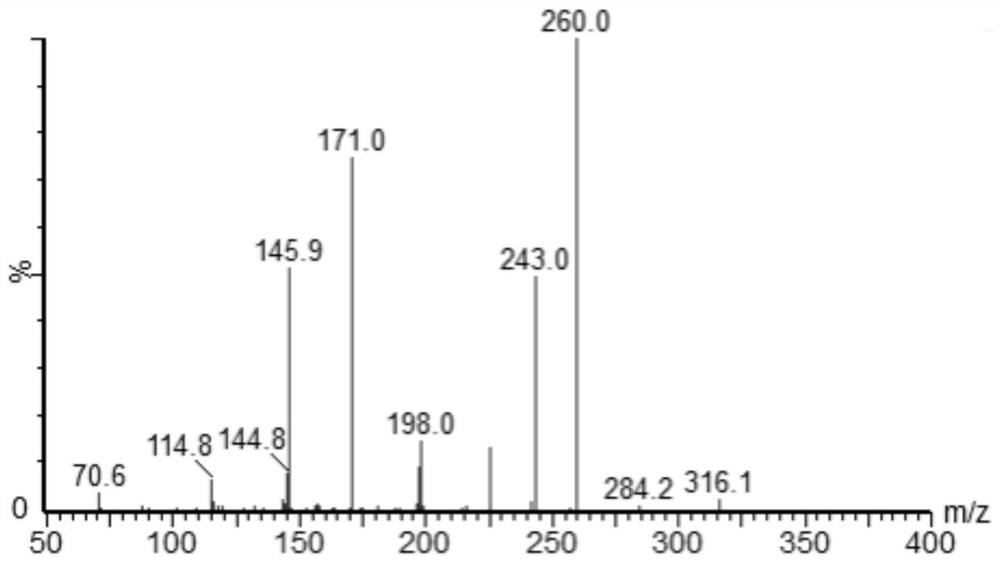
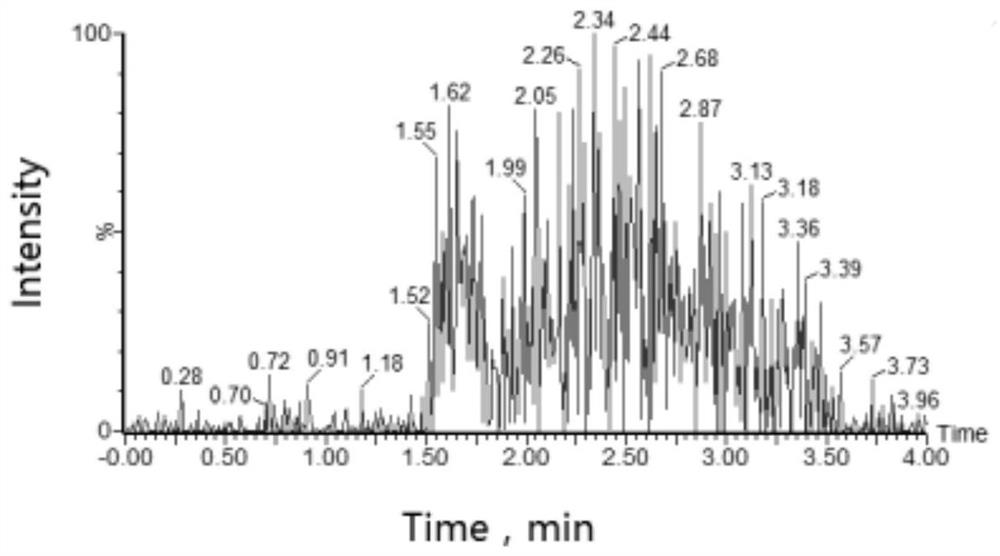
A method for quantitative analysis of plasma concentration of new compound wsj-557 in SD rat plasma
A WSJ-557, quantitative analysis technology, applied in the field of preclinical pharmacokinetics research in pharmaceutical analysis, to achieve the effect of less sampling, simple pretreatment and stable recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0036] 1. Required instruments and reagents
[0037] 1) Instrument
[0038] ACQUITY Ultra Performance LCTM ultra-high performance liquid chromatography (Waters, USA), Quattro micro TM API triple quadrupole tandem mass spectrometer equipped with electrospray ionization source (ESI source, Waters, USA).
[0039] 2) Reagent
[0040] Carbamazepine (purity 99.3%) was purchased from China Institute for the Control of Pharmaceutical and Biological Products; methanol and ammonium acetate were chromatographic grades; other chemical reagents were of analytical grade.
[0041] 2. Experimental part
[0042] 1) Plasma sample pretreatment method:
[0043] Plasma samples were obtained from mature SD rats at 7 to 9 weeks, with a body weight of 200g±50g; 0.3mL of blood was collected from the suborbital venous plexus, and the supernatant was obtained by centrifugation in a high-speed centrifuge; the sampling volume was 100μL.
[0044] Take 100 μL of plasma in a 10 mL glass test tube, add 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com