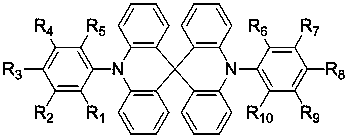
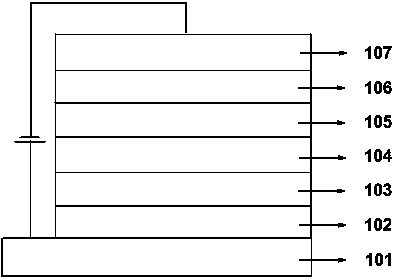
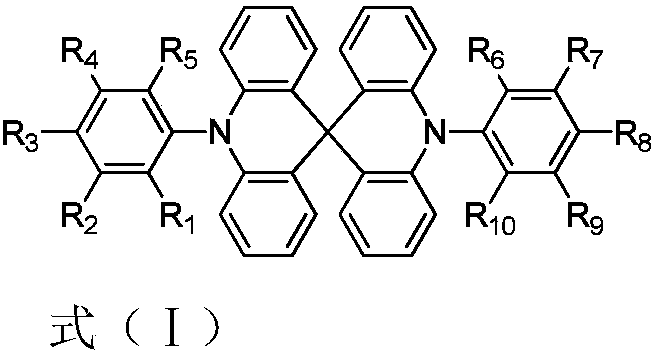
Thermally-activated delayed fluorescence materials and application thereof
A technology of thermally activated delayed and fluorescent materials, applied in the fields of luminescent materials, materials of organic semiconductor devices, organic chemistry, etc. problems such as bulk structural units, to achieve the effects of excellent thermal stability, excellent film stability, and excellent device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1, preparation of compound C01.
[0032]
[0033] Compound 1 was prepared by referring to the method described in Tetrahedron Letters, 2002, 43(32), 5521-5524.
[0034] Preparation of compound C01: In a 100mL three-necked flask, add compound 1 (2.08g, 6.0mmol), p-iodobenzonitrile (3.2g, 14mmol), potassium carbonate (2.07g, 15mmol), copper powder (0.65g), ortho 50g xylene, heat up to 140°C, keep the temperature for 15h, cool down to 25°C, pour the reaction solution into 150mL deionized water, add 150g tetrahydrofuran, filter with suction, collect the filtrate, separate the liquid, wash the organic phase with 150g deionized water, organic The solvent was removed under reduced pressure, and the obtained crude product was purified by silica gel column chromatography. The eluent was n-hexane:dichloromethane=1:2 (V / V). The obtained crude product of target C01 was further sublimated and purified using a chemical vapor deposition system. The sublimation temperature ...
Embodiment 2
[0035] Example 2, the preparation of compound C02.
[0036]
[0037] Using m-iodobenzonitrile as raw material, according to the method described in Example 1, compound C02 was prepared to obtain 1.3 g of the target object, high-resolution mass spectrometry, positive ion mode, molecular formula C 39 h 24 N 4 , theoretical value 548.2001, test value 548.2006, elemental analysis (C 39 h 24 N 4), theoretical value C: 85.38, H: 4.41, N: 10.21, measured value C: 85.40, H: 4.39, N: 10.21.
Embodiment 3
[0038] Example 3, preparation of compound C03.
[0039]
[0040] Using m-iodobenzonitrile as raw material, according to the method described in Example 1, compound C03 was prepared to obtain 1.5 g of the target object, high-resolution mass spectrometry, positive ion mode, molecular formula C 39 h 24 N 4 , theoretical value 548.2001, test value 548.2005, elemental analysis (C 39 h 24 N 4 ), theoretical value C: 85.38, H: 4.41, N: 10.21, measured value C: 85.40, H: 4.38, N: 10.22.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com