The preparation method of ponatinib
A technology of ponatinib and compounds, applied in the field of preparation of ponatinib, can solve the problems of high cost and unsatisfactory target product yield, and achieve the effects of wide temperature range, reduced synthesis cost and easy control of conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The invention provides a preparation method of ponatinib. The preparation method of described ponatinib comprises the steps:
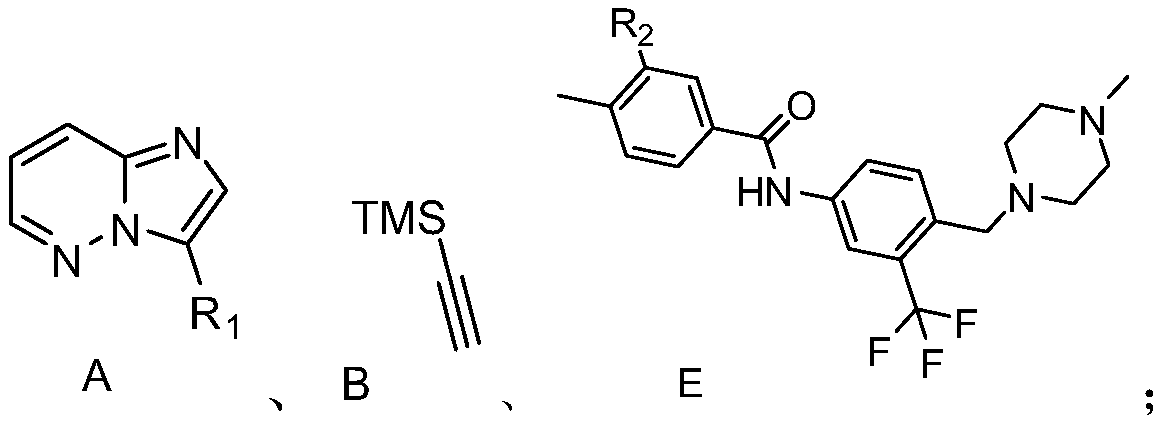
[0017] S01. Compounds A, B, and E of the following structural formula are provided, wherein the R contained in the compound A 1 is H, a halogen atom, and the R contained in the compound E 2 is a halogen atom:
[0018]
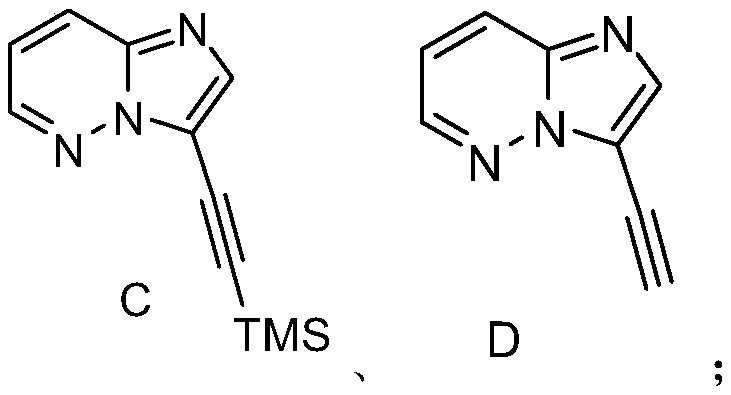
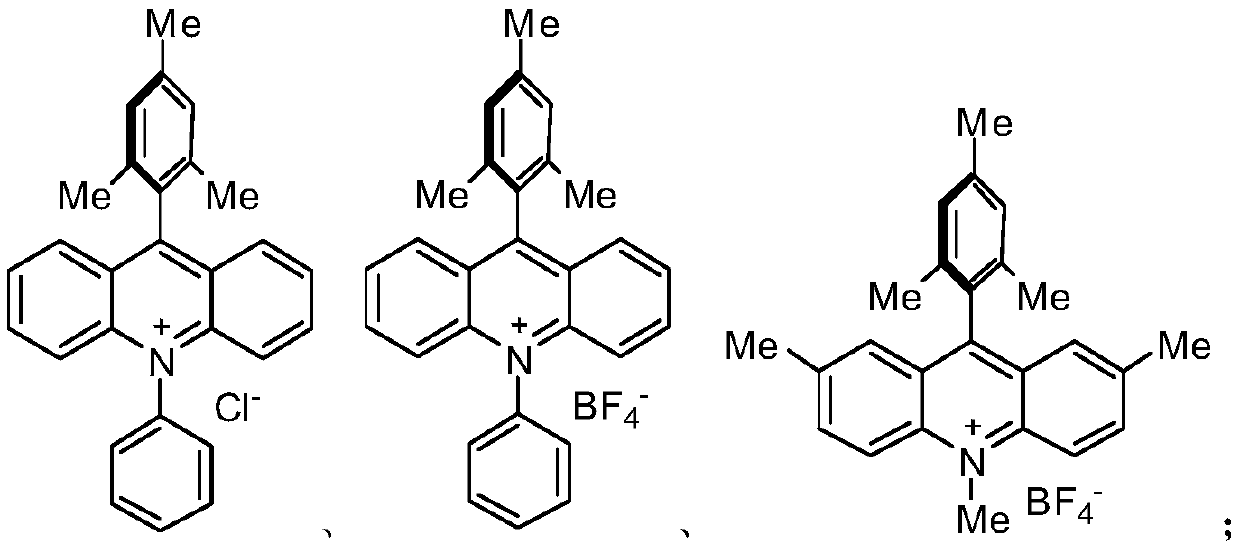
[0019] S02. In a protective atmosphere, the compound A and the compound B are subjected to light reaction in an organic solvent containing an acridinium salt photocatalyst, a copper salt, and an alkalinity to generate a compound C of the following structural formula;
[0020] S03. reacting the compound C with tetrabutylammonium fluoride to generate the compound D of the following structural formula;
[0021] S04. In a protective atmosphere, the compound D and the compound E are subjected to photoreaction in an organic solvent containing an acridinium salt photocatalyst, a copper salt, and an alkalinity to generate ponatinib...
Embodiment 2
[0050] This embodiment provides a preparation method of ponatinib. The preparation method of described ponatinib is as follows:
[0051] (1) Compound A and Compound B are added to an acetonitrile solvent containing CuCl and diisopropylamine, and illuminated by a blue LED light source for 24 hours to generate the target product Compound C; wherein, Compound A1mmol, Compound B1.2mmol, CuCl is 0.01 mmol, 0.05 mmol of acridinium salt, 2 equivalents of diisopropylamine, and 2 mL of acetonitrile solvent.
[0052] (2) Purifying Compound C and reacting it with tetrabutylammonium fluoride in tetrahydrofuran to generate the target product Compound D;
[0053] (3) Compound D and Compound E are added to an acetonitrile solvent containing CuCl and diisopropylamine, and illuminated by a blue LED light source for 10 hours to generate the target product ponatinib; wherein, Compound D1mmol, Compound E 1.1mmol, CuCl The concentration of the acridine salt is 0.03mmol, the acridinium salt is 0....
Embodiment 3
[0059] This embodiment provides a preparation method of ponatinib. The preparation method of described ponatinib is as follows:
[0060] (1) Compound A and Compound B are added to a DMF solvent containing CuBr and diisopropylamine, and illuminated by a blue LED light source for 48h to generate the target product Compound C; wherein, Compound A1mmol, Compound B1.2mmol, CuCl 0.05mmol , 0.05 mmol of acridinium salt, 2 equivalents of diisopropylamine, and 1.5 mL of DMF solvent.
[0061] (2) Purifying Compound C and reacting it with tetrabutylammonium fluoride in tetrahydrofuran to generate the target product Compound D;
[0062] (3) Compound D and Compound E are added to the DMF solvent containing CuBr and diisopropylamine, and illuminated by a blue LED light source for 10 hours to generate the target product ponatinib; wherein, compound D1mmol, compound E 1.1mmol, CuCl The concentration of DMF is 0.03mmol, that of acridinium salt is 0.03mmol, that of diisopropylamine is 1.2mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com