Preparation of human serum albumin modified black phosphorus quantum dots and applications of the quantum dots as a sensitizer
A technology of human serum albumin and black phosphorus quantum, which is applied in the field of tumor treatment, can solve problems such as difficult to cure, decline in the number and function of immune cells, easy metastasis and recurrence of tumors, etc., achieve low bioavailability, prolong the time of oxidation, improve The effect of blood compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Preparation and Characterization of Black Phosphorus Quantum Dots Modified by Human Serum Albumin
[0054] The black phosphorus quantum dots involved in the embodiments of the present invention are obtained by liquid-phase exfoliation of black phosphorus crystals. Its specific steps are as follows.
[0055] (1) Preparation of black phosphorus quantum dots: 25 mg of black phosphorus powder was added to a sealed conical tube containing 25 mL of organic solvent N-methylpyrrolidone solution, the probe was ultrasonicated every 2 seconds at intervals of 4 seconds, ultrasonic for 3 hours, and the ultrasonic frequency was from 19 to 25kHZ with a power of 1200W. The obtained black phosphorus solution was continuously ultrasonicated by an ultrasonic cleaner for 10 hours, the power was 300W, and the temperature was kept below 277K in an ice bath. Then centrifuge at 7000 rpm for 20 minutes to remove the precipitate, and then centrifuge at 12000 rpm for 20 minutes to obt...
Embodiment 2B
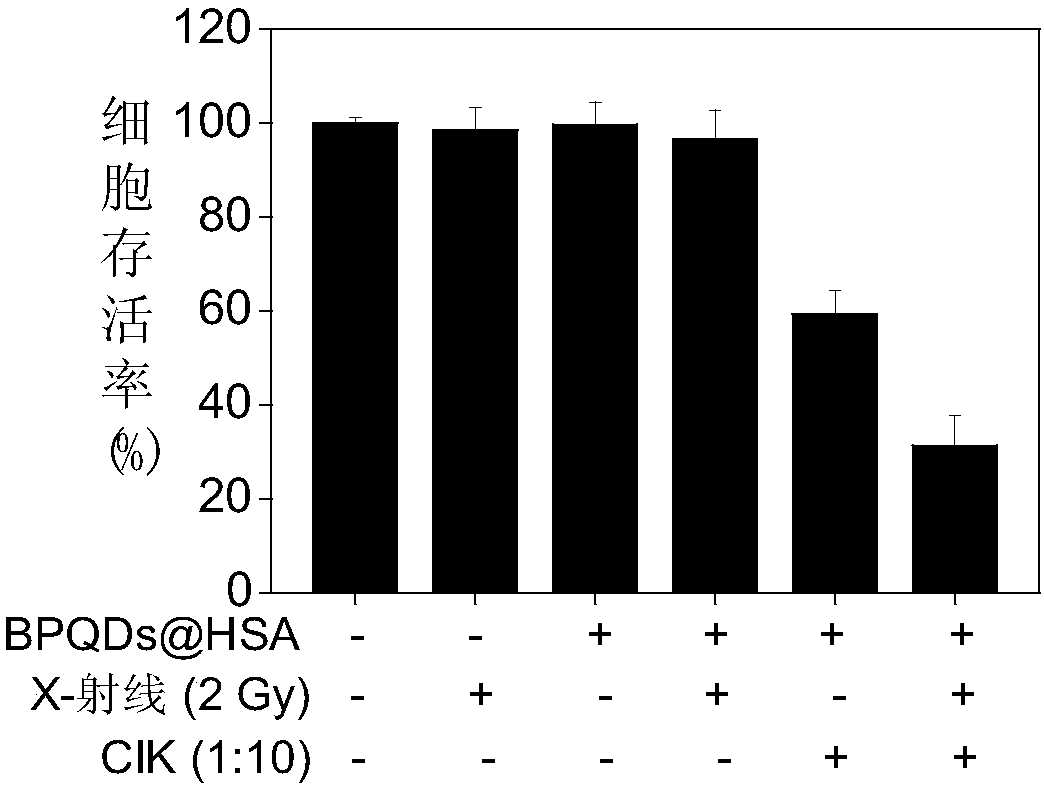
[0061] Example 2 Study on BPQDs@HSA synergy with CIK cell therapy and X-ray radiotherapy to enhance anti-hepatoma cell activity in vitro
[0062] The human serum albumin-modified black phosphorus quantum dots prepared in Example 1 cooperate with CIK cells and X-ray radiotherapy to enhance the anti-liver cancer effect of antibodies in vitro, and the specific steps are as follows.
[0063] (1) Toxicity study of BPQDs@HSA on CIK cells:
[0064] Take CIK cells in logarithmic growth phase (CIK cells are prepared with reference to Chinese patent application 2017100581925 "Application of Nano Selenium as a CIK Cell Sensitizer" Example 2) at a density of 20 × 10 4 cells / mL were inoculated in 96-well plates (100 μL / well), and the BPQDs@HSA prepared in Example 1 were prepared into final concentrations of 0.3, 0.6, 1.2, 2.5, 5 and 10 μg / mL, respectively, and added to CIK cells , respectively incubated for 24, 48 and 72 hours, using the CCK-8 kit (purchased from Japanese colleagues) to d...
Embodiment 3B
[0068] Example 3 Study on the mechanism of BPQDs@HSA synergizing with CIK cell therapy and X-ray radiotherapy to enhance anti-hepatic cancer in vitro
[0069] Apoptosis and cycle arrest are important factors that inhibit cell proliferation. In order to further detect the potential mechanism of human serum albumin-modified black phosphorus quantum dots BPQDs@HAS in cooperation with CIK cell therapy and X-ray radiotherapy to inhibit the proliferation of liver cancer cells prepared in Example 1, we used flow cytometry to analyze the potential mechanism of each treatment group Perform cell cycle analysis. The specific experimental steps are as follows:
[0070] First, HepG-2 liver cancer cells in the logarithmic growth phase were taken at a density of 2×10 4 cells / mL (6mL) were inoculated in a 6cm petri dish and allowed to grow adherently for 24 hours. Divided into six groups, including: blank control group, X-ray radiotherapy group, BPQDs@HAS nanomedicine group, BPQDs@HAS nano...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com