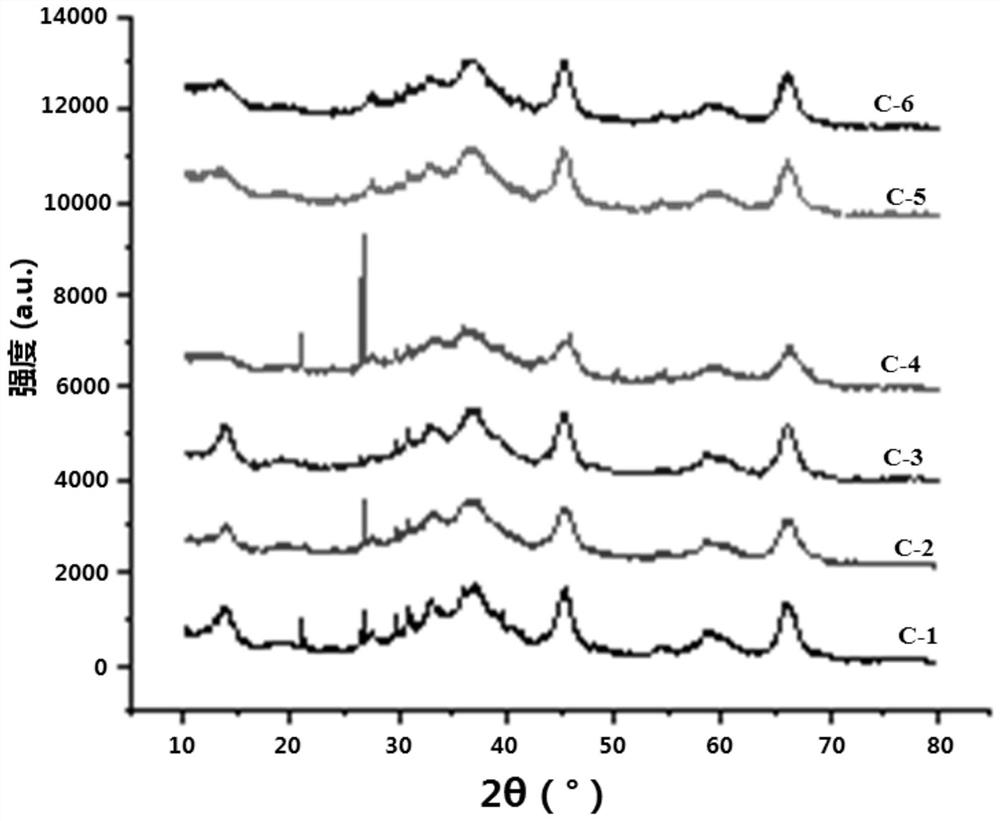
A low-temperature sulfur-tolerant shift catalyst and its preparation method
A sulfur-tolerant shift and catalyst technology, which is applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Strong hydration resistance, good stability, good low temperature activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Mix 186.7g of pseudo-boehmite, 21.84g of solid magnesium hydroxide and 17.1g of cobalt nitrate hexahydrate, and mix evenly in the mixing hopper of the extruder. Weigh 70g of water and add the above-mentioned solid material to mix, and continue to mix in the mixing hopper of the extruder for 1 hour. The material is extruded on an extruder, and the diameter of the formed sample is 3 mm. The molded samples were oven-dried at 120°C and fired at 500°C. The primary carrier is obtained after calcination. Take out 5g of the primary carrier, and impregnate 0.7345g of potassium silicate in equal volume on the primary carrier. After the impregnation is sufficient, the sample is oven-dried at 120°C and calcined at 500°C. After calcination, 0.6278g of ammonium molybdate was impregnated on the sample in an equal volume. After the impregnation was sufficient, the sample was oven-dried again at 120°C and then calcined at 500°C. That is, the low-temperature sulfur-tolerant shift cata...
Embodiment 2
[0046] Mix 186.7g of pseudo-boehmite, 21.84g of solid magnesium hydroxide and 17.1g of cobalt nitrate hexahydrate, and mix evenly in the mixing hopper of the extruder. Weigh 70g of water and add the above-mentioned solid material to mix, and continue to mix in the mixing hopper of the extruder for 1 hour. The material is extruded on an extruder, and the diameter of the formed sample is 3 mm. The molded samples were oven-dried at 120°C and fired at 500°C. The primary carrier is obtained after calcination. Take out 5g of the primary carrier, impregnate 0.8161g of potassium silicate in equal volume on the primary carrier, and after the impregnation is sufficient, dry the sample in an oven at 120°C and bake at 500°C. After calcination, 0.6977g of ammonium molybdate was impregnated on the sample in an equal volume. After the impregnation was sufficient, the sample was oven-dried again at 120°C and then baked at 500°C. That is, the low-temperature sulfur-tolerant shift catalyst C...
Embodiment 3
[0048] Mix 186.7g of pseudo-boehmite, 21.84g of solid magnesium hydroxide and 17.1g of cobalt nitrate hexahydrate, and mix evenly in the mixing hopper of the extruder. Weigh 70g of water and add the above-mentioned solid material to mix, and continue to mix in the mixing hopper of the extruder for 1 hour. The material is extruded on an extruder, and the diameter of the formed sample is 3mm. The molded samples were oven-dried at 120°C and fired at 500°C. The primary carrier is obtained after calcination. Take out 5g of the primary carrier, and impregnate 0.8977g of potassium silicate in equal volume on the primary carrier. After the impregnation is sufficient, the sample is oven-dried at 120°C and calcined at 500°C. After calcination, 0.7675g of ammonium molybdate was impregnated on the sample in equal volume. After the impregnation was sufficient, the sample was oven-dried again at 120°C and then calcined at 500°C. That is, the low-temperature sulfur-tolerant shift catalyst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com