Preparation method of 2-methyl-3-methoxyl-4-chloropyridine
A technology of methoxyl and chloropyridine, which is applied in the field of preparation of 2-methyl-3-methoxy-4-chloropyridine, can solve the problems of high reaction temperature, increased cost, and difficulty in handling, so as to improve the reaction rate , reduced dosage, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
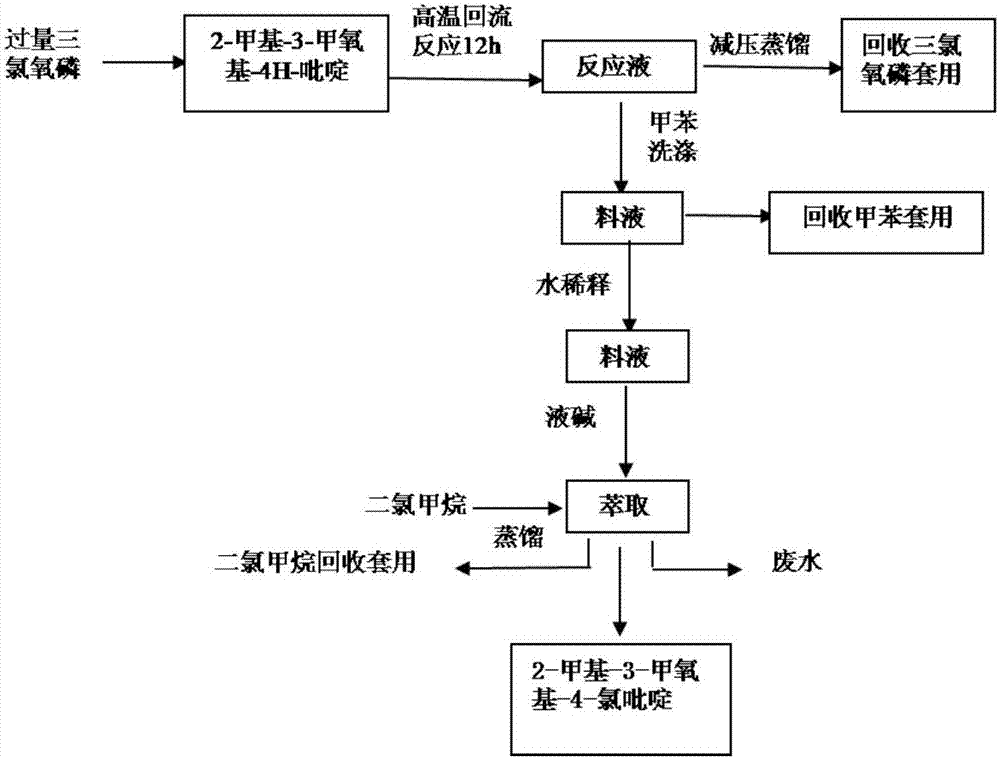
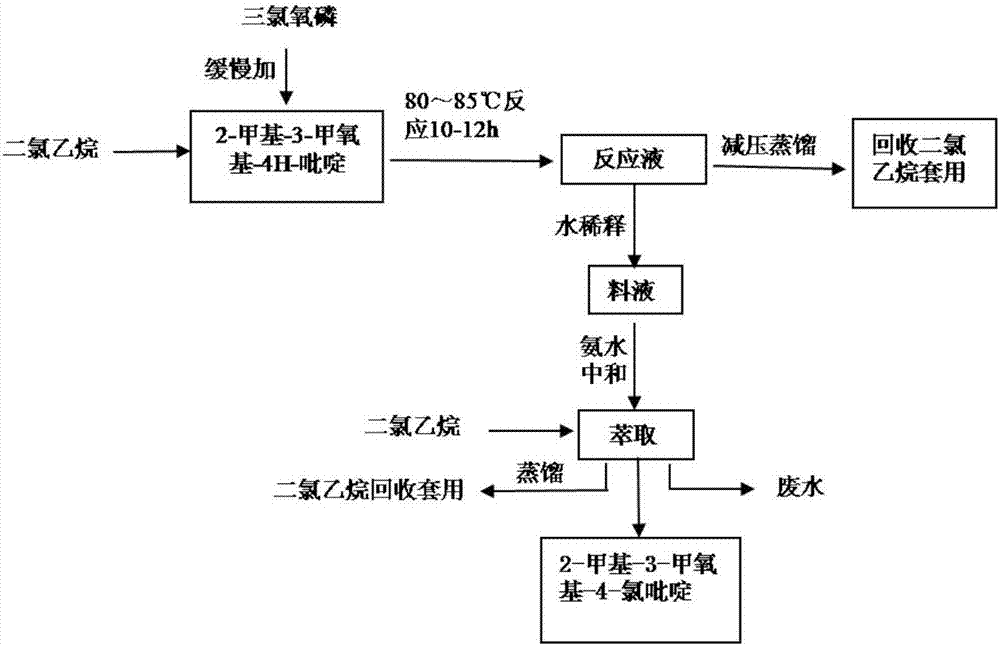
[0026] The preparation method of 2-methyl-3-methoxy-4-chloropyridine, see figure 2 , follow the steps below:
[0027] Step 1, adding 10kg of 2-methyl-3-methoxy-4H-pyridine into 75kg of dichloroethane, heating and dissolving to make the system solution clear;
[0028] Step 2, at 70°C, slowly add 12kg of phosphorus oxychloride to the system of step 1 under stirring, the dropping rate of phosphorus oxychloride is 4kg / h; then keep warm at 85°C for 10h to obtain a reaction solution;
[0029] Step 3: Distill the reaction liquid under reduced pressure at 85°C and 0.095Mpa, recover dichloroethane for later use, pass cooling water to cool the feed liquid after vacuum distillation to 18°C (room temperature), and slowly add it to 45kg of ice water Stir evenly, stir at 40°C for 2 hours for hydrolysis;
[0030] Step 4, cool down the hydrolyzed feed liquid to 10°C, add 35-55kg of ammonia water for neutralization, make the pH of the feed liquid 7-9, pour it into a separatory funnel and ...
Embodiment 2
[0032] The preparation method of 2-methyl-3-methoxyl group-4-chloropyridine specifically carries out according to the following steps:
[0033] Step 1, adding 13kg of 2-methyl-3-methoxy-4H-pyridine into 60kg of dichloroethane, heating and dissolving to make the system solution clear;
[0034] Step 2, at 70°C, slowly add 16kg of phosphorus oxychloride to the system of step 1 under stirring, the dropping rate of phosphorus oxychloride is 3.2kg / h; then keep warm at 83°C for 11h to obtain the reaction solution ;
[0035]Step 3, distill the reaction solution under reduced pressure at 83°C and 0.095Mpa, recover dichloroethane for later use, pass cooling water to cool the feed liquid after vacuum distillation to 20°C, slowly add it to 60kg of ice water and stir evenly, Stir at 35°C for 2h for hydrolysis;
[0036] Step 4, cool down the hydrolyzed feed solution to 5°C, add 35-55kg of ammonia water for neutralization, make the pH of the feed solution 7-9, pour it into a separatory fun...
Embodiment 3
[0038] The preparation method of 2-methyl-3-methoxyl group-4-chloropyridine specifically carries out according to the following steps:
[0039] Step 1, adding 15kg of 2-methyl-3-methoxyl-4H-pyridine into 50kg of dichloroethane, heating and dissolving to make the system solution clear;
[0040] Step 2, at 70°C, slowly add 18kg of phosphorus oxychloride to the system of step 1 under stirring, the dropping rate of phosphorus oxychloride is 3kg / h; then keep it warm at 80°C for 12h to obtain a reaction solution;
[0041] Step 3, distill the reaction solution under reduced pressure at 75°C and 0.095Mpa, recover dichloroethane for later use, pass cooling water to cool down the feed liquid after reduced-pressure distillation to 22°C (room temperature), and slowly add it to 75kg of ice water Stir evenly, stir at 30°C for 2 hours for hydrolysis;
[0042] Step 4, cool down the hydrolyzed feed liquid to 0°C, add 35-55kg of ammonia water for neutralization, make the pH of the feed liquid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com