Organic dye compounds with quinolinium ion skeleton structures and preparation method and application thereof
A technology of skeleton structure and organic dyes, which is applied in organic dyes, organic compounds/hydrides/coordination complex catalysts, organic chemistry, etc., can solve the problems of complex operation and difficulty in adjusting the luminescent properties of compounds, and achieve improvement The effect of electrical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
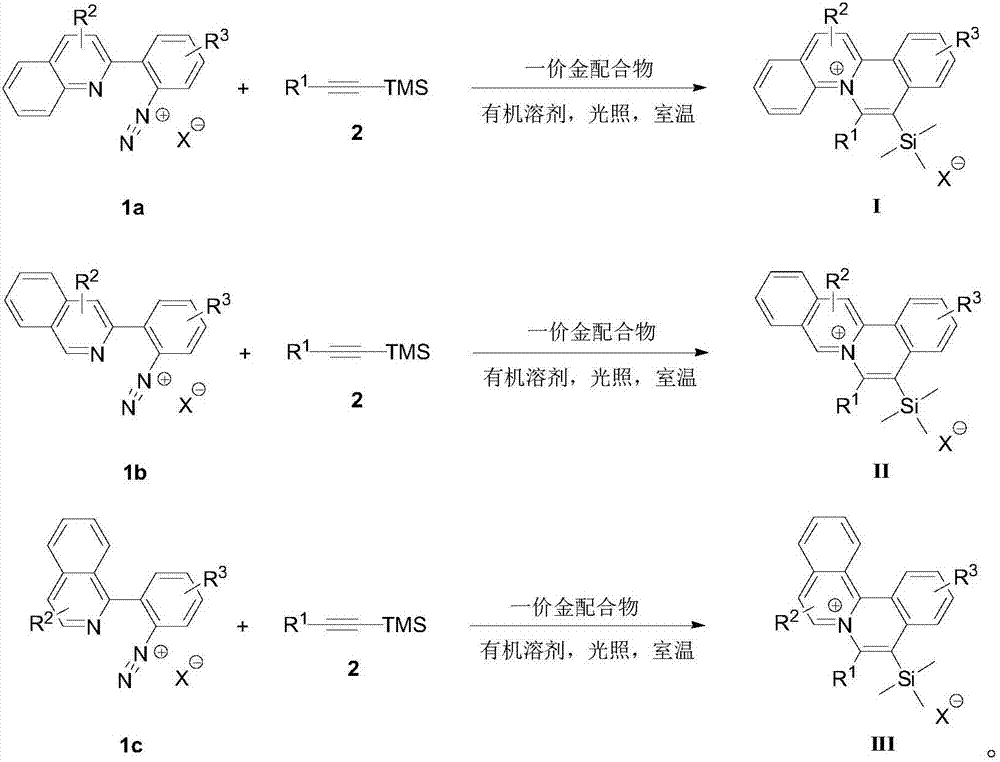
[0033] And, the embodiment of the present invention provides a method for preparing an organic dye compound with a quinolinium ion skeleton structure, comprising the following steps:
[0034] S01. Provide diazonium salts 1a, 1b, and 1c with quinoline structures shown in formulas 1a, 1b, and 1c, trimethylsilyl alkyne derivatives and monovalent gold complexes shown in formula 2, respectively;
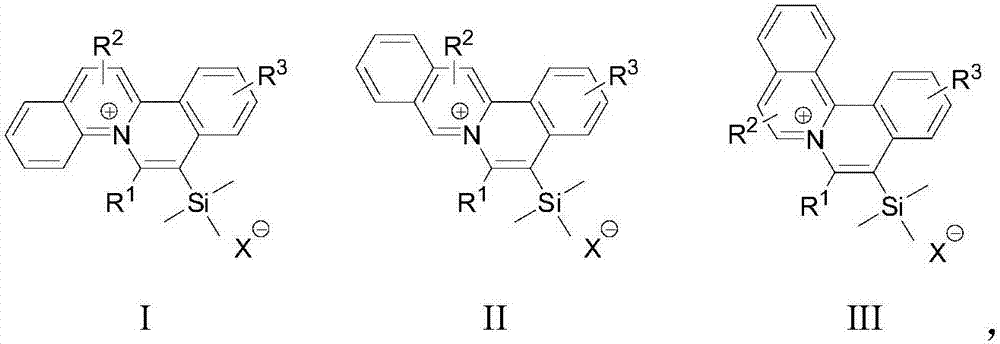
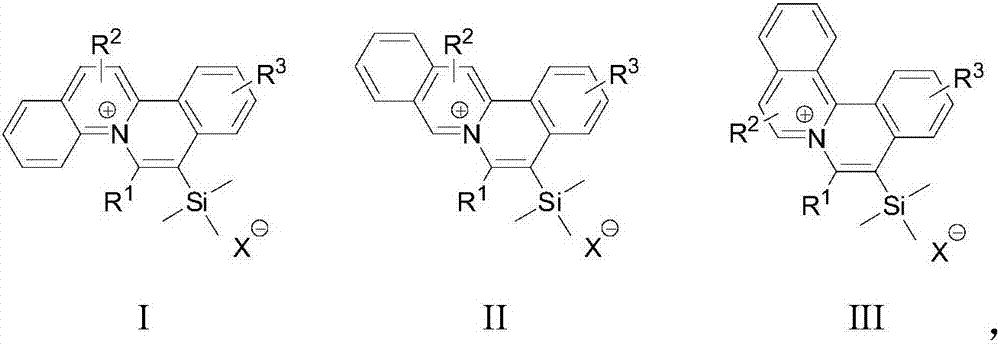
[0035] S02. Dissolve the 1a, 1b, and 1c respectively in an organic solvent, add the trimethylsilyl alkyne derivative 2, use the monovalent gold complex as a catalyst, and perform catalysis under visible light irradiation and room temperature conditions The cycloaddition reaction generates the general structural formula as the quinolinium ion skeleton structure organic dye compound shown in the following structures I, II, and III, and the reaction formula is as follows respectively,
[0036]
[0037] In the above step S01, the diazonium salts 1a, 1b, and 1c with quinoline structures rep...
Embodiment 1
[0058] Embodiment 1: the synthesis of organic dye I-1
[0059]
[0060] With quinoline structure diazonium salt 1a (0.6mmol), (4-methoxyphenylethynyl) trimethylsilane 2a (0.5mmol), Ph 3 PAuCl (0.05 mmol) and acetonitrile (5 mL) were mixed in a 20 mL glass test tube and sealed with a rubber stopper. The air in the test tube was removed by the freeze-thaw pump circulation method and flushed with nitrogen. After the mixture in the test tube returned to room temperature, the test tube was placed under the irradiation of a blue LED light source to react for 16 hours. After the reaction was completed, the solvent was removed, and the product was obtained as an orange solid after chromatographic separation, and the yield was 69%.
[0061] 1 H NMR (400MHz, MeOD) δ9.12(d, J=9.0Hz, 1H), 9.02(d, J=8.5Hz, 1H), 8.92(d, J=8.9Hz, 1H), 8.43(d, J =8.2Hz,1H),8.24(d,J=7.2Hz,1H),8.17(t,J=7.6Hz,1H),8.04(t,J=7.6Hz,1H),7.72(m,2H), 7.48-7.32(m,3H),7.06(d,J=8.7Hz,2H),3.90(s,3H),0.14(s,9H).
...
Embodiment 2
[0063] Embodiment 2: the synthesis of organic dye 1-2
[0064]
[0065] Refer to Example 1 for specific operation, yellow solid, yield 68%.
[0066] 1 H NMR (400MHz, MeOD) δ9.16(d, J=9.0Hz, 1H), 9.04(d, J=8.4Hz, 1H), 8.94(d, J=8.9Hz, 1H), 8.46(d, J =8.3Hz, 1H), 8.25(d, J=7.1Hz, 1H), 8.18(t, J=7.3Hz, 1H), 8.06(t, J=7.7Hz, 1H), 7.77-7.66(m, 2H ),7.66-7.58(m,1H),7.52(m,4H),7.39-7.26(m,1H),0.12(s,9H).
[0067] 13 C NMR(101MHz,MeOD)δ150.61,147.87,142.81,139.97,138.26,137.85,135.24,134.94,133.12,131.88,131.20,130.51,130.26,130.19,129.92,127.91,126.40,126.28,119.46,1.88.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com