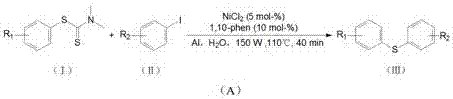
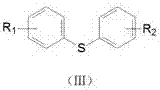
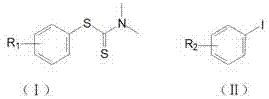Method for catalytically synthesizing diaryl thioether through phenylthiourea in aqueous phase under microwave radiation
A diaryl sulfide and microwave radiation technology, which is applied in the preparation of sulfide, organic chemistry, etc., can solve the problems of reduced reaction yield, high catalyst/ligand loading, high cost, etc., and achieve simple operation, high yield, The effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: 4-methyldiphenyl sulfide: 1.0 mmol of phenylthiourea, 1.2 mmol of 4-methyl iodobenzene, NiCl 2 5 mol-%, 1,10-phenanthroline 10 mol-%, Al powder 200 mol-%, water 3.0 mL, stirred for 5 min. Placed in a microwave reactor and heated to 100 °C under 150 W power for continuous reaction for 40 min. After the reaction was completed, it was cooled to room temperature, and the product was extracted with ethyl acetate, washed with saturated Na 2 SO 4 Dry and concentrate under reduced pressure, and the product is purified by column chromatography to obtain a colorless liquid with a yield of 86%.
Embodiment 2
[0029] Example 2: 5-methyldiphenyl sulfide: the preparation method is the same as in Example 1, and 1.2 mmol of 3-methyliodobenzene is added to the reaction vessel to obtain a colorless liquid with a yield of 83%.
Embodiment 3
[0030] Example 3: 6-methyldiphenyl sulfide: the preparation method is the same as that of Example 1, adding 1.2 mmol of 2-methyl iodobenzene into the reaction vessel to obtain a colorless liquid with a yield of 69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com