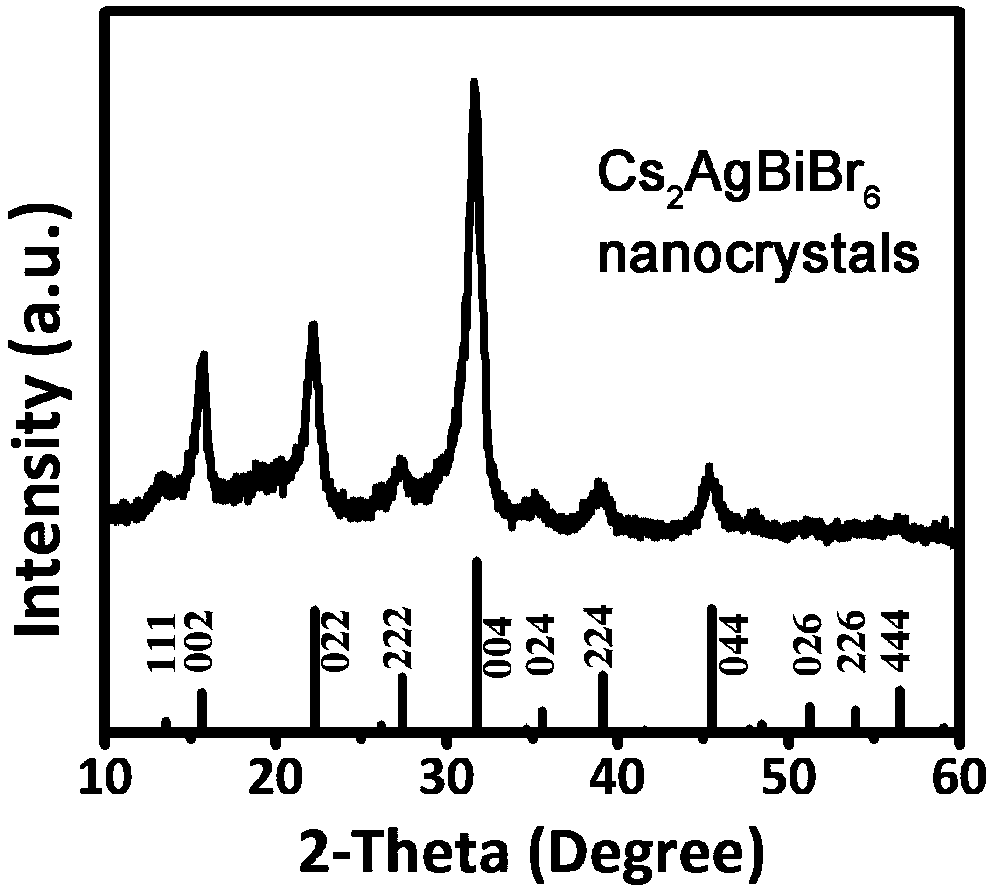
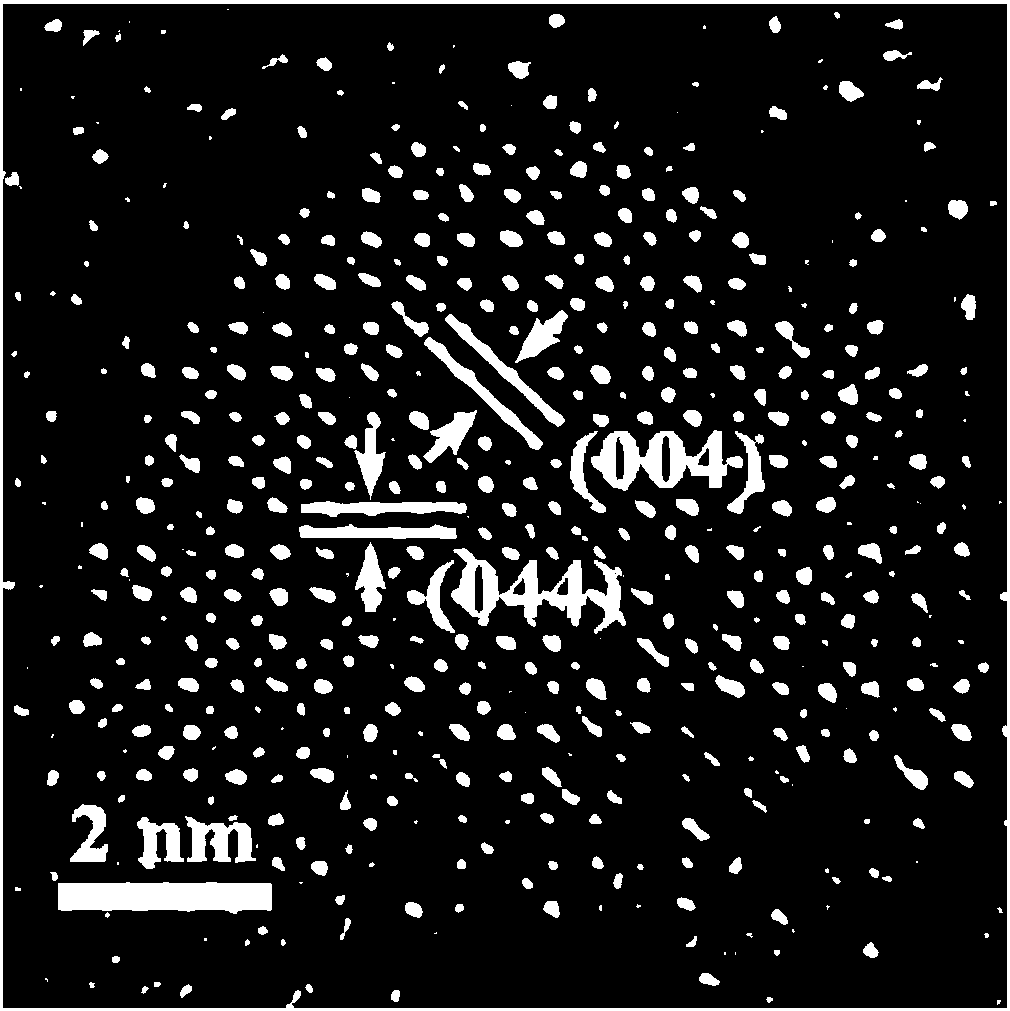
Preparation method of stable lead-free all-inorganic double perovskite A2BB'X6 nanocrystals
A double perovskite, nanocrystal technology, applied in inorganic chemistry, nanotechnology, chloride preparation, etc., can solve the problems of low stability, narrow size distribution of nanocrystals, good dispersibility, etc., and achieve high stability and method. Simple, good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A stable lead-free double perovskite A 2 BB’X 6 A method for preparing nanocrystals, comprising the steps of:
[0035] Step 1, 45mg BiBr 3 , 17mg AgNO 3 , 0.1mL HBr, 1mL oleic acid, 1mL oleylamine and 4mL octadecene were mixed simultaneously, heated and stirred under vacuum at 120°C for 1h to ensure that the water and gas in the system were removed, and then heated under N 2 Heated to 200°C under protection to obtain a clear and transparent solution.
[0036] Step 2, 0.814g Cs 2 CO 3 , 40mL of octadecene and 2.5mL of oleic acid were added to a 100mL three-neck flask at the same time, and then stirred under vacuum at 120°C for 1h to make Cs 2 CO 3 Fully react with oleic acid to obtain a clear and transparent octadecene solution of cesium oleate; 0.8mL of the cesium oleate solution obtained is quickly injected into the precursor solution prepared in step 1), and after reacting for 5s, the system ice bath is cooled to room temperature.
[0037] Step 3, centrifuge at...
Embodiment 2
[0041] Synthesize Cs using the preparation method described 2 AgBr 6 Nanocrystals, and then washed with a low-polarity solvent to remove the ligands on the surface of the nanocrystals, applied to the photocatalytic reduction of CO 2 .
[0042] To the 4mLCs prepared in embodiment 1 2 AgBr 6 Add 2 mL of absolute ethanol to the nanocrystal dispersion, shake and wash the mixed system for 30 s, and then centrifuge at 12,000 rpm for 5 min to obtain a yellow precipitate in the lower layer. The washing process is repeated twice. Finally, the surface ligand was washed and treated with Cs 2 AgBr 6 Nanocrystalline.
[0043] The Cs obtained in this example 2 AgBr 6 Nanocrystals were at 55% relative humidity, 70mW·cm -2 The stability test was carried out under the light conditions and the high temperature environment of 100 ℃.
[0044] pass Figure 5 The XRD results show that Cs 2 AgBr 6 The nanocrystals are stable for more than 90 days at 55% relative humidity. At 70mW·cm -...
Embodiment 3
[0047] A stable lead-free double perovskite A 2 BB’X 6 A method for preparing nanocrystals, comprising the steps of:
[0048] Step 1, 45mg BiBr 3 , 17mg AgNO 3 , 0.1mL HBr, 1mL oleic acid, 1mL oleylamine and 4mL octadecene were mixed simultaneously, heated and stirred under vacuum at 120°C for 0.5h to ensure that the water and gas in the system were removed, and then heated under N 2 Heated to 200°C under protection to obtain a clear and transparent solution.
[0049] Step 2, 0.814g Cs 2 CO 3 , 40mL of octadecene and 2.5mL of oleic acid were added to a 100mL three-neck flask at the same time, and then stirred under vacuum at 120°C for 1h to make Cs 2 CO 3 Fully react with oleic acid to obtain a clear and transparent octadecene solution of cesium oleate. Take 0.8 mL of the obtained cesium oleate solution and quickly inject it into the precursor solution prepared in step 1), and after reacting for 5 seconds, quickly cool the system down to room temperature in an ice bath...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com