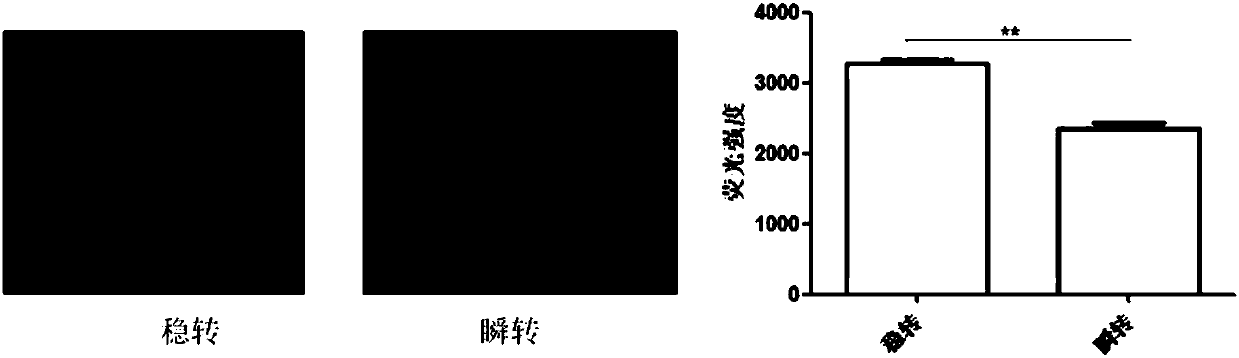
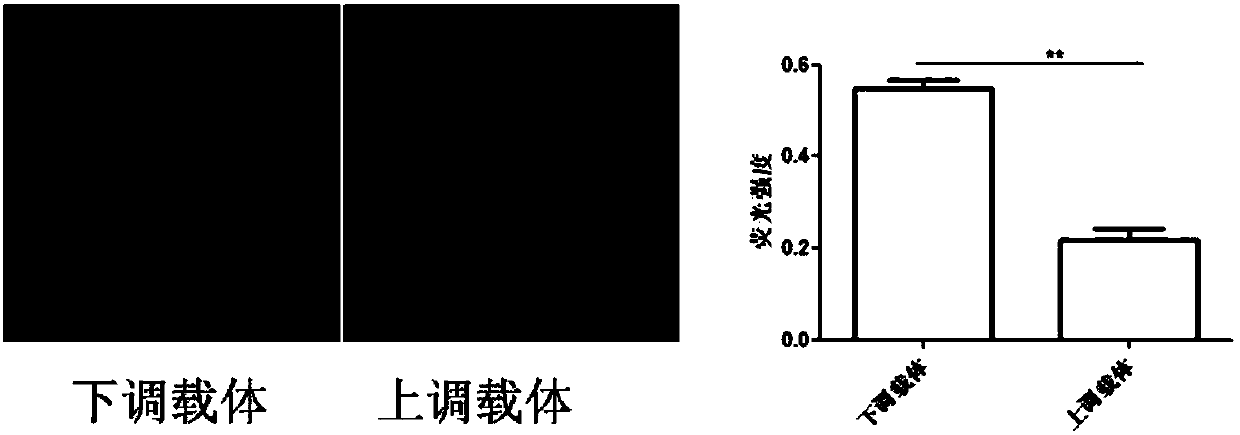
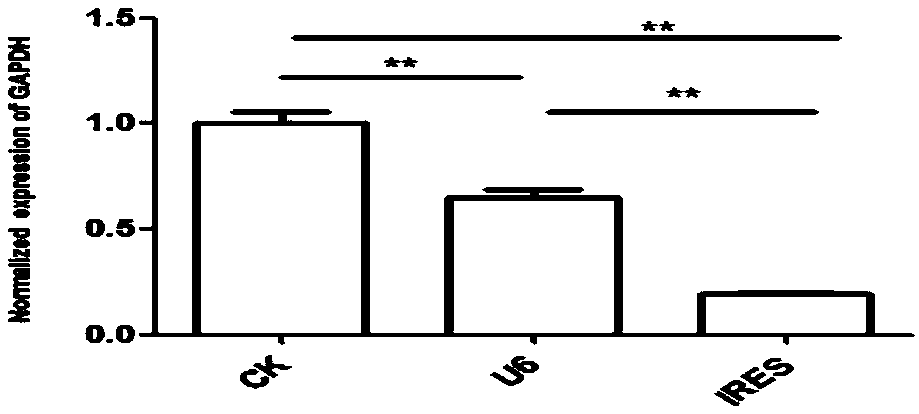
Vector for assaying in-vitro cell proliferation and dynamic in-vitro cell proliferation assay method
A technology of cell proliferation and dynamic detection, applied in the field of cell detection, can solve the problems of not being able to obtain the true state of living cells, not being able to detect and analyze cells, and destroying cells, so as to reduce inactivated cells and cell fragments, with small errors and repeatability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0118] Example 2: Commonly used cell proliferation detection method--MTT method
[0119] 1. Collect the logarithmic phase cells and adjust the concentration of the cell suspension to 1X 10 4 . The diluted cell suspension was added to a 96-well plate, and 200 μl was added to each well. Each sample was repeated 3-5 times, and a blank control well with medium only was set, and the edge wells were filled with sterile PBS. A total of seven 96-well plates were inoculated.
[0120] 2. Put it in a CO2 incubator, 5% CO2, and incubate at 37°C. Thereafter, the medium was changed every 2 days.
[0121] 3. On the second day, take out a 96-well plate, add 20 μl of MTT solution (5 mg / ml, ie 0.5% MTT) to each well, and continue culturing for 4 hours.
[0122] 4. Carefully aspirate and discard the supernatant in the wells, add 150 μl DMSO to each well, shake on a shaker at low speed for 10 minutes, and the crystals are fully dissolved.
[0123] 5. On the ELISA instrument, select 490nm to...
Embodiment 3
[0126] Example 3: Commonly used cell proliferation detection kit--CCK-8 method
[0127] 1. Collect logarithmic phase cells and adjust the concentration of cell suspension to 1X10 4 . The diluted cell suspension was added to a 96-well plate, and 200 μl was added to each well. Each sample was repeated 3-5 times, and a blank control well with medium only was set, and the edge wells were filled with sterile PBS. A total of seven 96-well plates were inoculated.
[0128] 2. Put it in a CO2 incubator, 5% CO2, and incubate at 37°C. Thereafter, the medium was changed every 2 days.
[0129] 3. On the second day, take out a 96-well plate, add 20 μl Cell Counting kit solution to each well, and continue to incubate for 4 hours.
[0130] 4. On the ELISA instrument, select 450nm to measure the absorbance value of each well, and calculate the average value.
[0131] 5. After every 24 hours, take out a 96-well plate and repeat steps 3-4. 6. Draw the cell growth curve with time as the X a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com