Preparation method of votistine hydrobromide midbody
A technology of vortioxetine hydrobromide and intermediates, which is applied in the field of drug synthesis, can solve the problems of unfavorable safety and environmental protection, long reaction time, poor product quality, etc., to reduce safety and environmental risks, suitable for industrial production, and product quality controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of 1-tert-butoxycarbonyl-4-[2-(2,4-dimethylphenylsulfanyl)phenyl]-3,5-dioxopiperazine (Intermediate C)
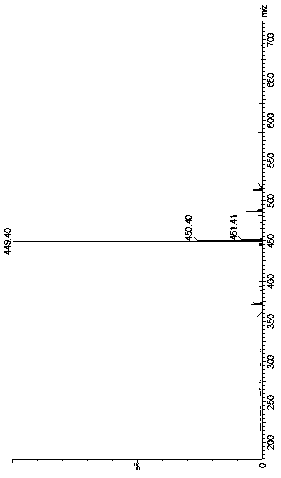
[0034] Put 233.9g of dicyclohexylcarbodiimide, 4.26g of 4-dimethylaminopyridine, and 500ml of dichloromethane into a 3L three-necked flask, stir at 20~30°C for 30min, and dissolve 100g of intermediate B in 600ml of dichloromethane In the medium, control the temperature at 20~30°C and slowly drop it in. After the dropping, react for 60~70min. After the reaction is complete, add 1000ml of 2N hydrochloric acid to wash, wash with 1000ml of saturated sodium bicarbonate, evaporate the organic layer to dryness under reduced pressure, add 1500ml of ethanol, and heat up to Stir at 73~80°C for 15~25min, cool down to 0~5°C, filter with suction, and dry under reduced pressure at 40°C to obtain 160.3g of off-white crystalline powder with a purity of 99.354% and a yield of 86.18%.
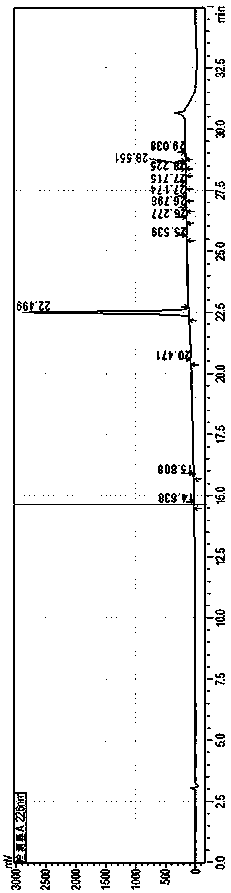
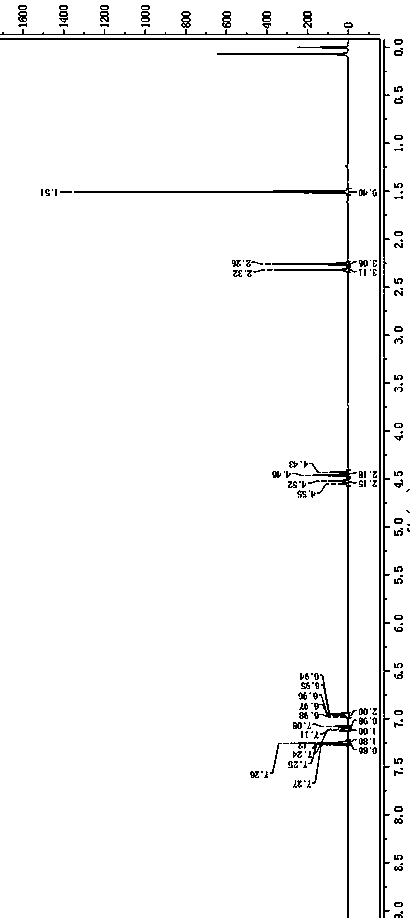
[0035] HPLC Detection Conditions
[0036]
[0037]
Embodiment 2
[0038] Example 2 Preparation of 1-tert-butoxycarbonyl-4-[2-(2,4-dimethylphenylsulfanyl)phenyl]-3,5-dioxopiperazine (Intermediate C)
[0039] Put 145g of bisisopropylcarbodiimide, 4.50g of 4-dimethylaminopyridine, and 500ml of ethyl acetate into a 3L three-necked flask, stir at 20~30°C for 30min, and dissolve 106g of intermediate B in 600ml of ethyl acetate , control the temperature at 20~30℃ and slowly drop it into the three-necked flask. After the dropping, react for 60~70min. , heated to 60-65°C, stirred for 15-25min, cooled to 0-5°C, filtered with suction, and dried under reduced pressure at 40°C to obtain 165.8g of off-white crystalline powder with a purity of 99.318% and a yield of 89.14%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com