Fusion protein and applications thereof
A fusion protein, binding region technology, applied in the direction of immunoglobulin, immunoglobulin superfamily, animal/human protein, etc., can solve problems such as the application of restriction marker genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Example 1. Expression of fusion protein FR806
[0125] In this embodiment, eGFP is selected as a fluorescent marker for analysis and testing, and eGFP is enhanced green fluorescent protein. Select F2A as the self-cleaving sequence. F2A is a core sequence of 2A (or "self-cleaving polypeptide 2A") from foot-and-mouth disease virus, which has the "self-cleaving" function of 2A; The partial amino acid sequence (SEQ ID NO:32) of type 1 (FOLR1) and the partial sequence (SEQ ID NO:28) of EGFR were expressed as fusion protein FR806 (SEQ ID NO:44), and the signal peptide of FOLR1 was selected as the signal peptide. The following genetic engineering operations were performed using standard methods known to those skilled in the art. The preparation method of the nucleotide (SEQ ID NO:1) of eGFP-F2A-FR806 is as follows:
[0126] SEQ ID NO:1
[0127] (eGFP is shown in bold, F2A is shown underlined, FR SP (folate receptor signal peptide) is shown in bold and underlined, epitope 80...
Embodiment 2
[0161] Synthesis and titration of embodiment 2, CH12-biotin
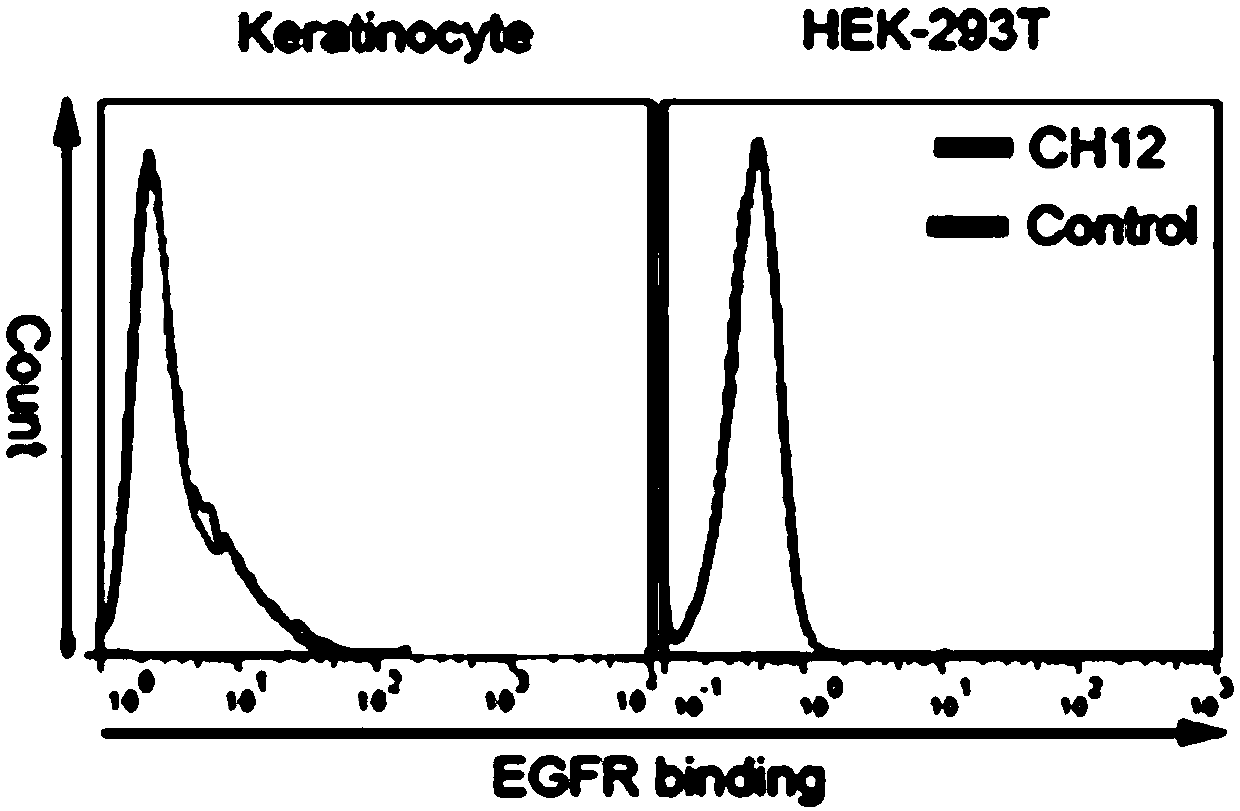
[0162] The CH12 antibody was labeled with biotin. Dilute CH12 antibody to 2.5mg / ml, PBS pH7.4, labeling volume 1.6ml; take 1mg of Sulfo-NHS-LC-Biotin (Thermo Company), add 180ul of ultrapure water to dissolve; take 79ul of Biotin and add to 1.6 ml of CH12 antibody, react overnight. Use PD-10 desalting column (GE company, USA) to desalt, replace in PBS 5% glycerol buffer solution, obtain CH12-Biotin, OD280 / 1.45 concentration is 0.77mg / ml.
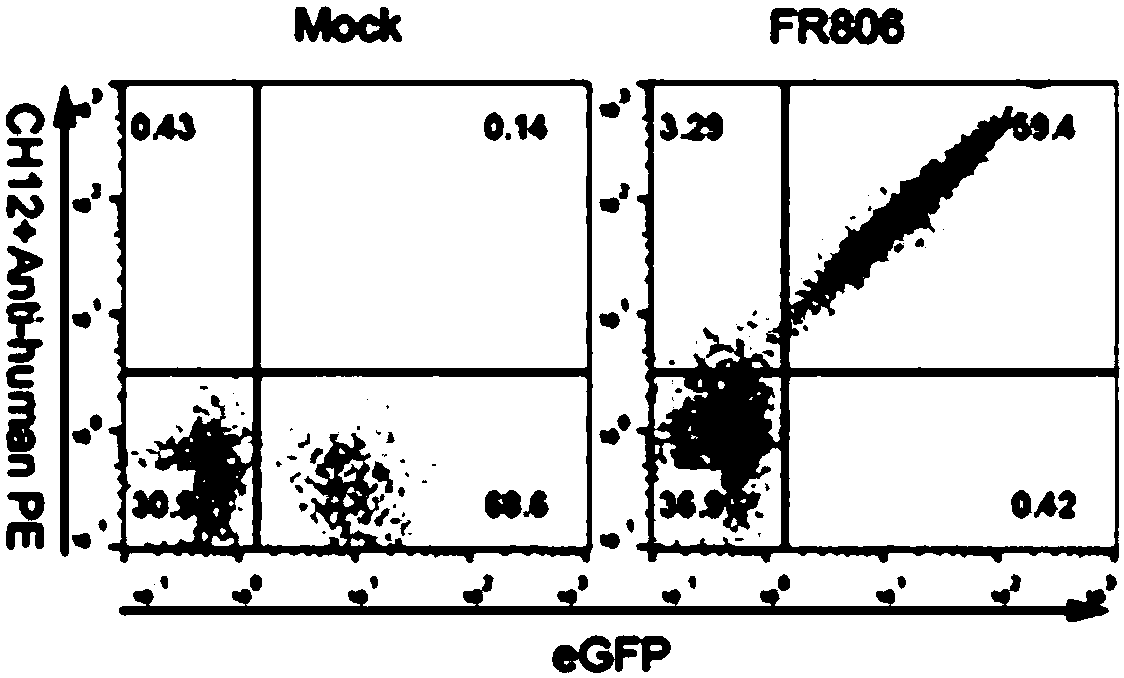
[0163] Dilute CH12-biotin to different concentrations (100 μg / ml, 10 μg / ml, 1 μg / ml, 0.1 μg / ml, 0.01 μg / ml, 0 μg / ml) with PBS containing 1% FBS, respectively, and express eGFP-F2A -The T cells of FR806 were incubated for 45 minutes, then washed with PBS, the secondary antibody was diluted with PE-SA (ebioscience company) 1:300 medium, and after adding the resuspended cells, incubated for 45 minutes. After washing twice with PBS, flow cytometry analysis, the results are as follo...
Embodiment 3
[0164] Example 3. Using CH12-biotin to sort FR806 positive T cells
[0165] Take 1×10 7 T cells expressing eGFP-F2A-FR806 were washed with PBS, incubated with CH12-biotin (10 μg / ml, diluted in PBS containing 1% FBS) for 45 min at 4°C, then washed with PBS, and anti-Biotin sorting magnetic beads (purchased from Meitian Nii Company), according to the steps given by the sorting magnetic bead product, the T cells of FR806 were sorted out. Take an appropriate amount of cells before and after sorting, and perform flow cytometry analysis. The results are as follows: Figure 4 As shown, T cells expressing FR806 can be effectively sorted out by anti-Biotin sorting magnetic beads after being combined with CH12-biotin, and the sorting positive rate reaches 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com