Genistein derivative and preparation method and application thereof
A technology of genistein and its derivatives, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., can solve the problems of high toxicity and side effects and limitations of bone marrow suppression, and achieve good bioavailability, low side effects, good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of genistein derivatives, comprising the steps of:
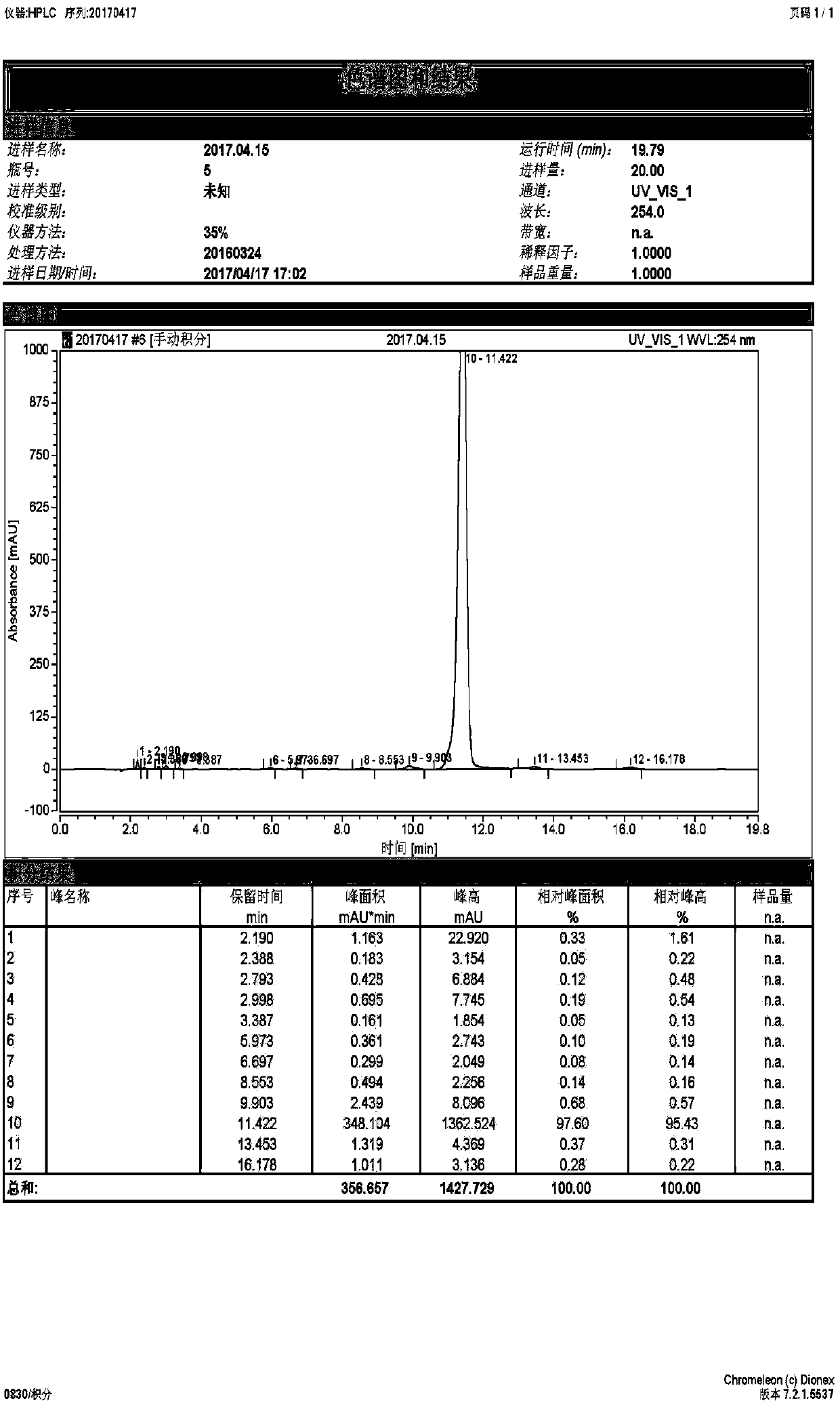
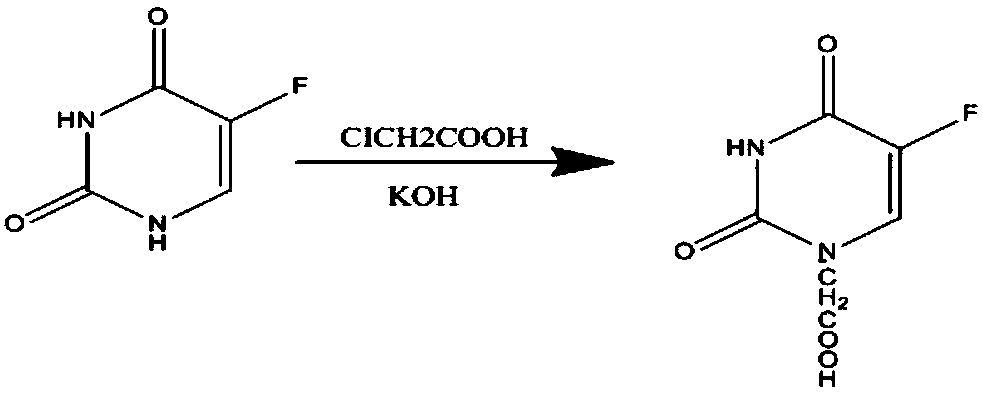
[0033] (1) Synthesis of 5-fluorouracil-1-acetic acid: at first, add 1.6g 5-fluorouracil and 30ml of alkaline aqueous solution of 10wt% in the three-necked flask that is equipped with reflux condenser and magnetic force stirring, then slowly add to the three-necked flask Add 4ml of 0.525g / mL chloroacetic acid solution dropwise. After the dropwise addition, heat and stir in a water bath at 60°C for 5 hours. After the reaction, cool the reaction solution to room temperature, adjust the pH value to 2, and then place it in a refrigerator at 4°C to cool overnight. , suction filtration, and drying to obtain needle-like crystal 5-fluorouracil-1-acetic acid, the specific reaction formula is as follows:
[0034]
[0035] (2) Preparation of genistein derivatives: take 0.5 g of 5-fluorouracil-1-acetic acid prepared in the above step (1) in a three-necked flask, then add 0.6 g of genistein, 0.6 g of catalys...
Embodiment 2
[0038] A preparation method of genistein derivatives, comprising the steps of:
[0039] (1) Synthesis of 5-fluorouracil-1-acetic acid: at first, add 1.6g 5-fluorouracil and 40ml of alkaline aqueous solution of 10wt% in the three-necked flask that is equipped with reflux condenser and magnetic force stirring, then slowly add to the three-necked flask Add 4ml of 0.65g / mL chloroacetic acid solution dropwise. After the dropwise addition, heat and stir in a water bath at 60°C for 5 hours. After the reaction, cool the reaction solution to room temperature, adjust the pH value to 2, and then place it in a refrigerator at 4°C to cool overnight. , suction filtration, and drying to obtain needle-like crystal 5-fluorouracil-1-acetic acid, the specific reaction formula is as follows:
[0040]
[0041] (2) Preparation of genistein derivatives: Take 0.5 g of 5-fluorouracil-1-acetic acid prepared in the above step (1) in a three-necked flask, then add 1.1 g of genistein, 1.0 g of catalyst...
Embodiment 3
[0044] A preparation method of genistein derivatives, comprising the steps of:
[0045] (1) Synthesis of 5-fluorouracil-1-acetic acid: at first, add 1.6g 5-fluorouracil and 30ml of alkaline aqueous solution of 10wt% in the three-necked flask that is equipped with reflux condenser and magnetic force stirring, then slowly add to the three-necked flask Add 4ml of 0.75g / mL chloroacetic acid solution dropwise. After the dropwise addition, heat and stir in a water bath at 60°C for 5 hours. After the reaction, cool the reaction solution to room temperature, adjust the pH value to 2, and then place it in a refrigerator at 4°C to cool overnight. , suction filtration, and drying to obtain needle-like crystal 5-fluorouracil-1-acetic acid, the specific reaction formula is as follows:
[0046]
[0047] (2) Preparation of genistein derivatives: Take 0.45 g of 5-fluorouracil-1-acetic acid prepared in the above step (1) in a three-necked flask, then add 0.6 g of genistein, 0.6 g of catalyst ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com