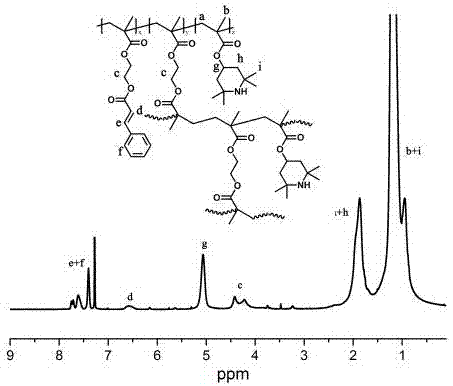
Photo-crosslinking stabilized hyperbranched free radical polymer and preparation method and application thereof
A free radical and stabilization technology, applied in the direction of electrical components, circuits, battery electrodes, etc., can solve the problems of machinability, non-rechargeable battery life, etc., achieve good mechanical properties, avoid battery life decline, molecular weight and the effect of increasing the glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Preparation method
[0046] A photocrosslinkable stabilized hyperbranched free radical polymer prepared by the following method:
[0047] (1) Add 1.624g of 2,2,6,6-tetramethyl-4-piperidinyl methacrylate and 0.208g of methacryloyloxyethyl cinnamate in sequence to a 20mL dry ammeter tube , 0.16g of ethylene glycol dimethacrylate, 44.4mg of 4-cyano-4-(propyl trithiocarbonate)-pentanoic acid and 5.2mg of azobisisobutyronitrile (ie its mole ratio of 90:10:10:2:0.4), and 8 mL of tetrahydrofuran as a solvent, nitrogen was passed through to remove oxygen, and the tube was sealed in vacuum. After the reaction was continued for 18 h under heating at 60° C., the reaction solvent was removed by rotary evaporation to obtain the hyperbranched polymer hb-poly(TMPM-co-CEMA).
[0048] (2) Get 1g of gained hyperbranched polymer and 0.854g m-chloroperoxybenzoic acid (i.e. the mol ratio of piperidinyl unit and m-chloroperoxybenzoic acid is 1:1.1), be dissolved in 250mL dichloromethane...
Embodiment 2
[0054] 1. Preparation method
[0055] According to the same preparation method as in Example 1, but the feeding was changed to 1.624g of 2,2,6,6-tetramethyl-4-piperidinyl methacrylate, 0.208g of methacryloxyethyl cinnamon ester, 40 mg of ethylene glycol dimethacrylate, 44.4 mg of 4-cyano-4-(propyltrithiocarbonate)-pentanoic acid and 5.2 mg of azobisisobutyronitrile, which The molar ratio is 90:10:2.5:2:0.4. After post-treatment under the same conditions as in Example 1, a hyperbranched radical polymer (hb-2) capable of photocrosslinking and stabilization was obtained.
[0056] 2. Product characteristics and physical and chemical properties
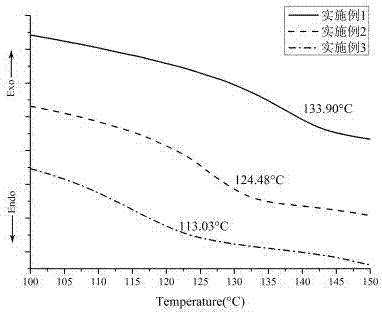
[0057] (1) if figure 2 As shown, the glass transition temperature of the obtained photocrosslinkable and stabilized hyperbranched radical polymer (hb-2) is 124.48°C.
[0058] (2) Using the same method as in Example 1 to prepare an electrode, the measured electrode capacity is 83mAh, and the coulombic efficiency is nearly 100% and the ...
Embodiment 3
[0060] 1. Preparation method
[0061] According to the same preparation method as in Example 1, but the feeding was changed to 1.624g of 2,2,6,6-tetramethyl-4-piperidinyl methacrylate, 0.208g of methacryloxyethyl cinnamon ester, 16 mg of ethylene glycol dimethacrylate, 44.4 mg of 4-cyano-4-(propyltrithiocarbonate)-pentanoic acid and 5.2 mg of azobisisobutyronitrile, which The molar ratio is 90:10:1:2:0.4. After post-treatment under the same conditions as in Example 1, a hyperbranched radical polymer (hb-3) capable of photocrosslinking stabilization was obtained.
[0062] 2. Product characteristics and physical and chemical properties
[0063] (1) if figure 2 As shown, the glass transition temperature of the obtained photocrosslinkable and stabilized hyperbranched radical polymer (hb-3) is 113.03°C.
[0064] (2) Using the same method as in Example 1 to prepare an electrode, the measured electrode capacity is 86mAh, and the coulombic efficiency is nearly 100% and the capaci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com