Chicken luteinizing hormone releasing hormone recombinant antigen castrating vaccine and preparation method therefor
A luteinizing hormone and hormone-releasing technology, applied in the direction of luteinizing hormone-releasing hormone, vaccines, contraceptive vaccine components, etc., can solve problems such as chicken luteinizing hormone-releasing hormone recombinant antigen castration vaccines that have no effect, and achieve castration Good effect, avoid trouble, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Construction of cLHRH-RA-RS1 strain recombinant expression plasmid
[0030] 1. Design and artificial synthesis of cLHRH gene
[0031] The design of gene fragments is based on the amino acid sequence of chicken luteinizing hormone releasing hormone (cLHRH) and the corresponding Escherichia coli preferred codons, and two complementary cLHRH gene sequences are designed in combination with the characteristics of the pET-32c plasmid itself. Under the premise of ensuring that the reading frame is correct after inserting the vector, add two restriction sites, Ncol and Xhol, at the 5' end and 3' end respectively, and add two protective bases before the restriction site, and use DNA automatic The synthesizer synthesizes the gene, and the gene sequence is as follows:
[0032] P1:5'-GGCCATGGCTGAACACTGGTCCTACGGTCTGCGTCCGAACGGCGGTGAGCACTGGTCTTACGGCCTGCAGCCGGGTGGCGGTGAACATTGGTCTTACGGTCTGCGCCCGAACTAATGAACTCGAGCA-3' (SEQ ID NO: 1)
[0033] P2:5'-TGCTCGAGTTCATTAGTTCGGGCGCAGA...
Embodiment 2
[0047] Embodiment 2 prepares original bacterial liquid
[0048] According to the protein expression characteristics and the immunogenicity of the expressed protein, the original strains used for the development of chicken luteinizing hormone releasing hormone recombinant antigen castration vaccine were screened, and the specific process was as follows:
[0049] 1. Conversion
[0050] The cLHRH / pET-32c recombinant plasmid was transformed into Escherichia coli BL21 (DE3) competent bacteria, and at the same time, Escherichia coli was transformed with the pET-32c plasmid as a negative vector control. Take 200 μl of transformed bacteria liquid, smear it on LB agar plate containing Amp50 μg / ml, use untransformed competent bacteria as negative control, and culture at 37°C for 18 hours. Store at 2-8°C. After the colonies are picked and amplified and screened, the best colonies are selected to establish the original seed batch.
[0051] 2. Prepare the original bacterial liquid
[00...
Embodiment 3
[0053] The determination of embodiment 3 optimum inoculum amount
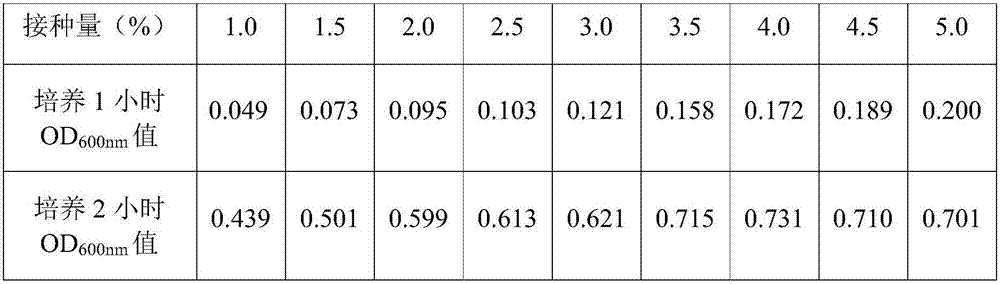
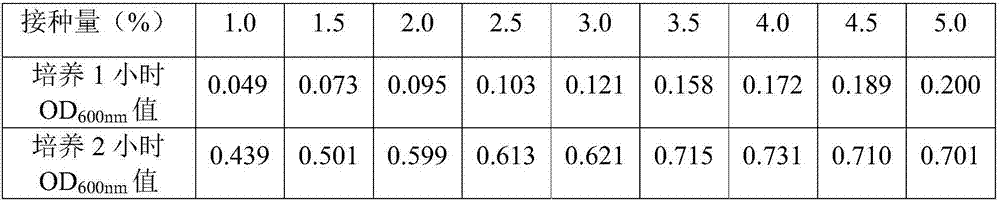
[0054] The original bacterial liquid obtained in Example 2 was inoculated into a 1000ml Erlenmeyer flask containing 250ml Amp-LB liquid medium, and the inoculation amounts were respectively 1.0%, 1.5%, 2.0%, 2.5%, 3.0%, 3.5%, 4.0%, 4.5% %, 5.0%, repeated three times under the same conditions, respectively, the first group, the second group, the third group, all cultured with shaking at 36-37°C and 230r / min. Record the growth curve of the bacterial culture, and take samples 1 hour and 2 hours after the culture, and measure the OD 600nm The results are shown in Table 1-3.
[0055] Table 1 Inoculum size and OD 600nm Value relations (first set)
[0056]
[0057] Table 2 Inoculum size and OD 600nm Value Relationships (Second Group)
[0058]
[0059] Table 3 Inoculum size and OD 600nm Value Relationships (Group 3)
[0060]
[0061] The experimental results show that when the inoculation amount of each...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com