Transaminase mutant and application thereof
A technology of transaminase and mutant, which is applied to the transaminase mutant and its application field, can solve the problem that the transaminase is not suitable for industrial production, and achieve the effects of improving enzyme specificity, reducing cost and improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
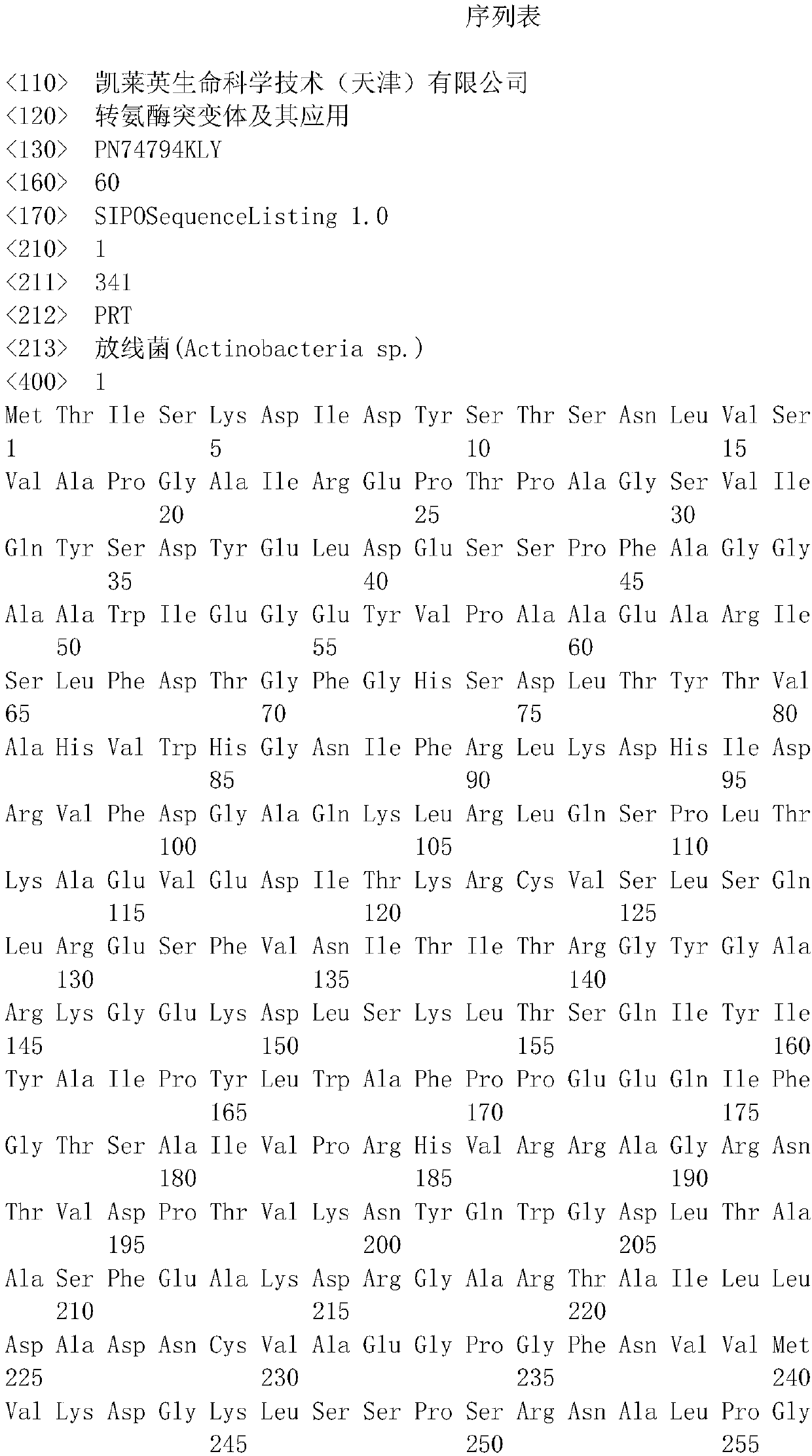
[0032] According to a typical embodiment of the present invention, a recombinant plasmid is provided. The recombinant plasmid contains any one of the above DNA molecules. The DNA molecules in the above recombinant plasmids are placed in appropriate positions of the recombinant plasmids, so that the above DNA molecules can be replicated, transcribed or expressed correctly and smoothly.
[0033] Although the qualifier used in the present invention is "contains" when limiting the above-mentioned DNA molecule, it does not mean that other sequences irrelevant to its function can be arbitrarily added to both ends of the DNA sequence. Those skilled in the art know that in order to meet the requirements of recombination operations, it is necessary to add suitable restriction endonuclease cutting sites at both ends of the DNA sequence, or additionally add start codons, stop codons, etc., therefore, if using A closed-ended statement will not truly cover these situations.
[0034] The ...
Embodiment 1
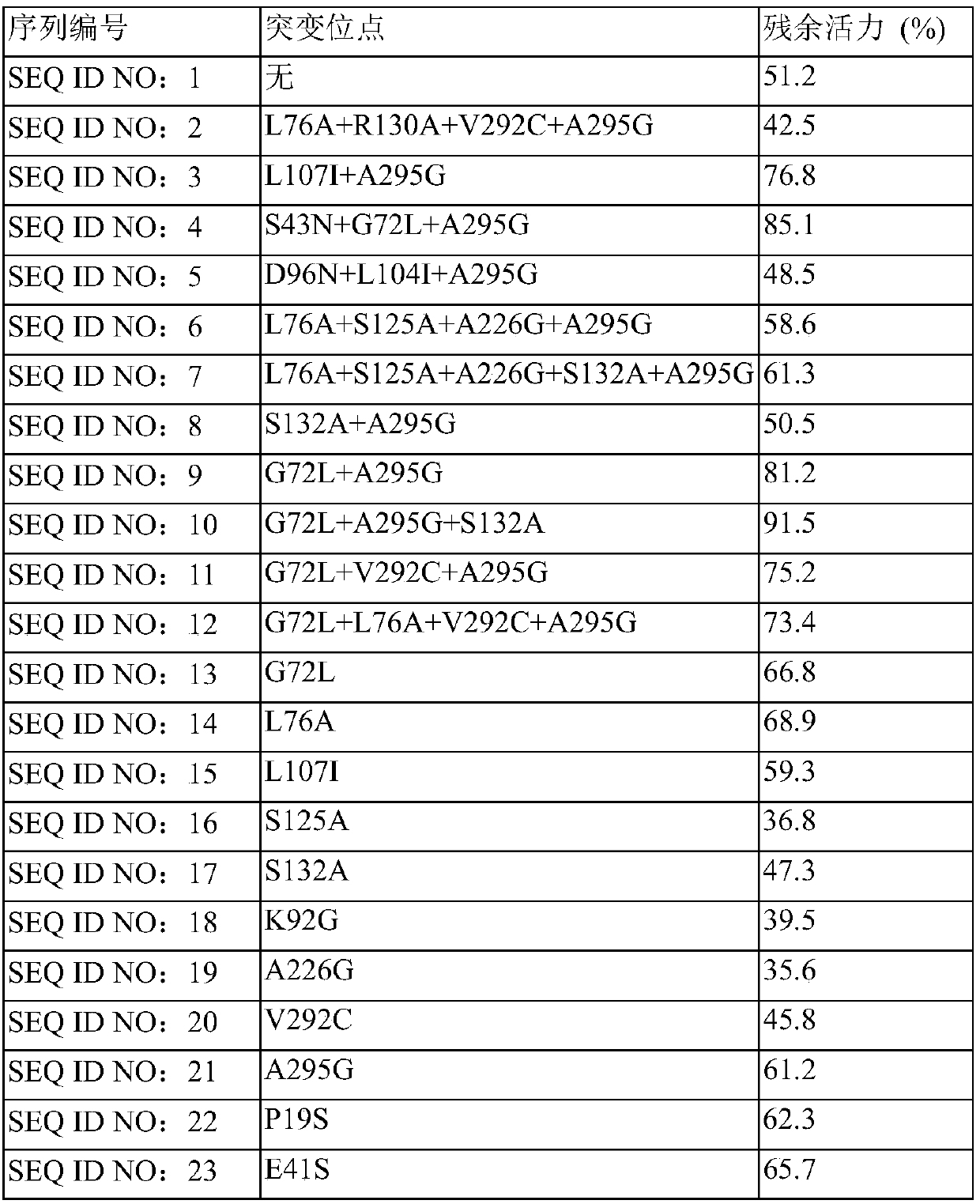
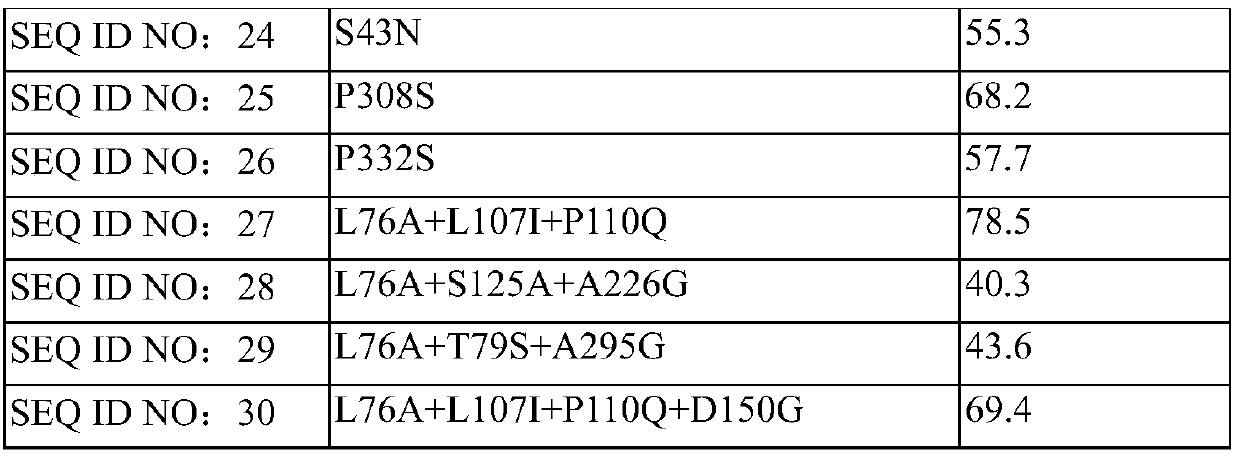
[0045] In a 10mL reaction bottle, add 40mg of isopropylamine into 1mL of 0.2M phosphate buffer solution, adjust the pH to 7.0~7.5, add transaminase SEQ ID NO: 1 with a mass of 120mg, add 0.4mg of pyridoxal phosphate, mix well and drop into 40 mg dissolved in 0.2 mL DMSO The pH of the system is 7.0-7.5, and the mixture is stirred at a constant temperature of 35°C±3°C for 16h. The conversion rate of the system was detected by GC. According to the above steps, the reaction data of the mutant transaminase whose sequence number is SEQ ID NO: 13-26 is as follows in Table 1:
[0046] Table 1
[0047] serial number
mutation site
16h
Enzyme dosage
SEQ ID NO: 1
none
21.3%
3wt
SEQ ID NO: 13
G72L
36.8%
2wt
SEQ ID NO: 14
L76A
47.1%
2wt
SEQ ID NO: 15
L107I
47.7%
2wt
SEQ ID NO: 16
S125A
38.9%
2wt
SEQ ID NO: 17
S132A
52.8%
2wt
SEQ ID NO: 18
K92G
39....
Embodiment 2
[0051] Generally speaking, the performance of mutants with single-point mutations is difficult to be significantly different from that of the mother parent, and the combination of mutation points can obtain better mutants. Therefore, the mutation sites are randomly recombined by DNA shuffling, a mutation library is established, and then screened to try to obtain better mutants.
[0052]DNA shuffling is the sexual recombination of genes at the molecular level. A group of homologous genes were digested into random fragments with nuclease I, and these random fragments were used to form a library, which were used as primers and templates for PCR amplification. Template exchange and genetic recombination occur when fragments of one gene copy serve as primers for another gene copy.
[0053] Enzyme solution preparation method: centrifuge the 96-well plate to remove the supernatant medium, add 200 μl of enzymatic hydrolysis solution (lysozyme 2 mg / mL, polymyxin 0.5 mg / mL, pH=7.0) int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com