Preparation method for branched alkanes in range of gasoline, aviation kerosene and diesel oil
A technology of aviation kerosene and branched alkanes, which is applied in the preparation of liquid hydrocarbon mixtures, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problems of rising costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-14
[0026] 1. Preparation of catalyst:
[0027] 1) Preparation of solid acid catalysts: Nafion and Amberlyst resins, Y-type molecular sieves, montmorillonite K-10 and KSF are commercial catalyst products purchased directly.
[0028] Phosphorylated zirconium oxide (ZrP) catalyst is mixed with 1mol / L zirconium oxychloride and ammonium dihydrogen phosphate aqueous solution at a volume ratio of 2:1, and the obtained precipitate is washed and filtered repeatedly, dried at 120°C for 10 hours, and then placed in Calcined at 400°C for 4h.
[0029]2) Preparation of solid base catalysts: alkaline earth oxides (MgO, CaO, SrO) and rare earth oxides (La 2 o 3 , CeO 2 ) respectively by the corresponding nitrate in N 2 Calcined under atmosphere for 8h.
[0030] Magnesium aluminum hydrotalcite is the mass of 0.093mol Mg(NO 3 ) 2 ·6H 2 O and 0.0465mol Al(NO 3 ) 3 9H 2 O is dissolved in 100ml of water, and the solution is mixed with 0.219mol NaOH and 0.0565mol NaOH in a water bath at 70°...
Embodiment 13
[0041] Sedimentation and precipitation method: Prepare 0.0175M nickel nitrate solution and divide it into two parts A and B in equal volume. Add silica, H-ZSM-5, H-MOR, silicon-aluminum composite carrier, H-β molecular sieve, oxidation One kind of aluminum and an appropriate amount of concentrated nitric acid, 0.0525M urea was added to B, B was slowly added dropwise to A in a water bath at 80°C, the temperature was raised to 90°C, stirred for 10 hours, filtered and washed, dried overnight at 80°C, and air roasted at 500°C for 2 hours. The calcined catalyst was reduced in situ with hydrogen at 500 °C for 2 h in a fixed bed. (see table 1, embodiment 14-18)
Embodiment 14-18
[0042] Table 1 Supported metal A / X type bifunctional catalyst
[0043]
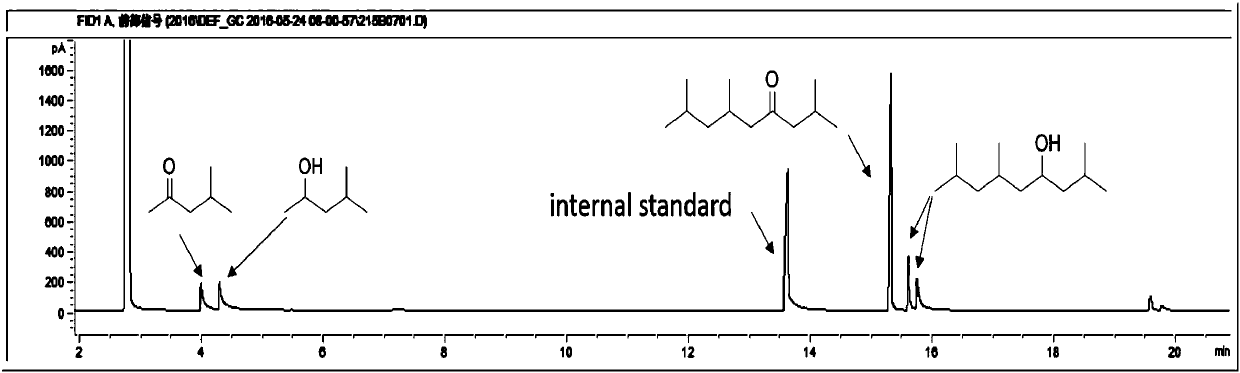
[0044] 2 Self-condensation reaction: In a fixed-bed reactor, put 1.0g of catalyst into the reaction tube, keep the hydrogen pressure in the reactor at 0.6MPa, and the hydrogen flow rate at 150mL / min. Pump into the reactor at 0.05 mL / min. The reaction results are shown in Table 2 and Table 3.
[0045] Table 2 methyl isobutyl ketone self condensation reaction result
[0046]
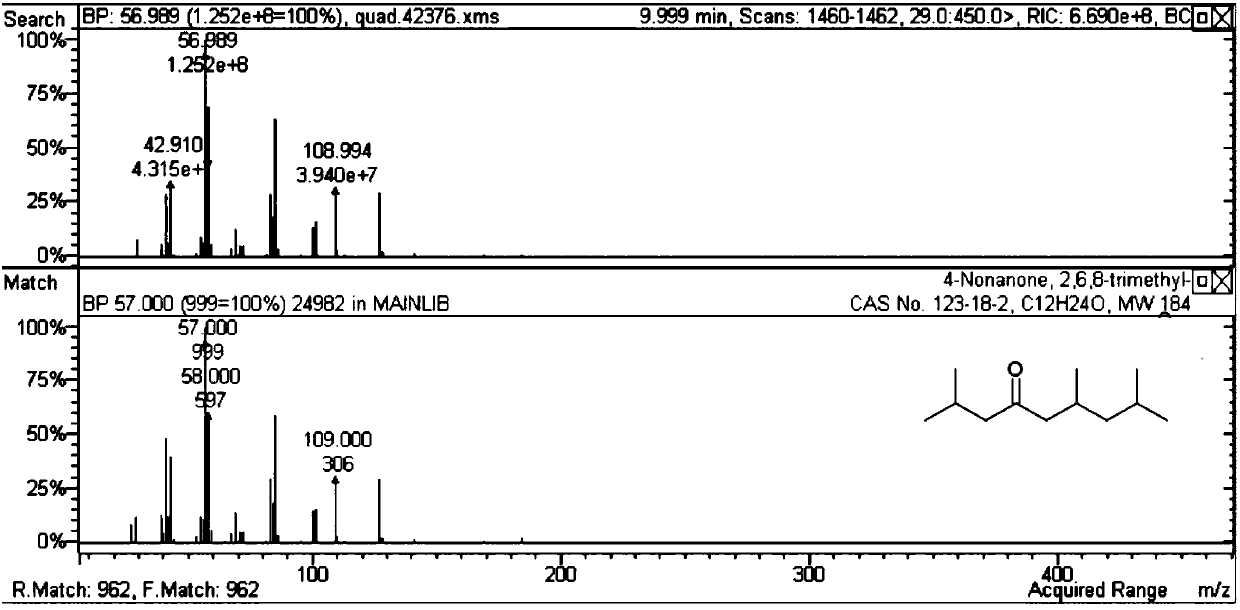
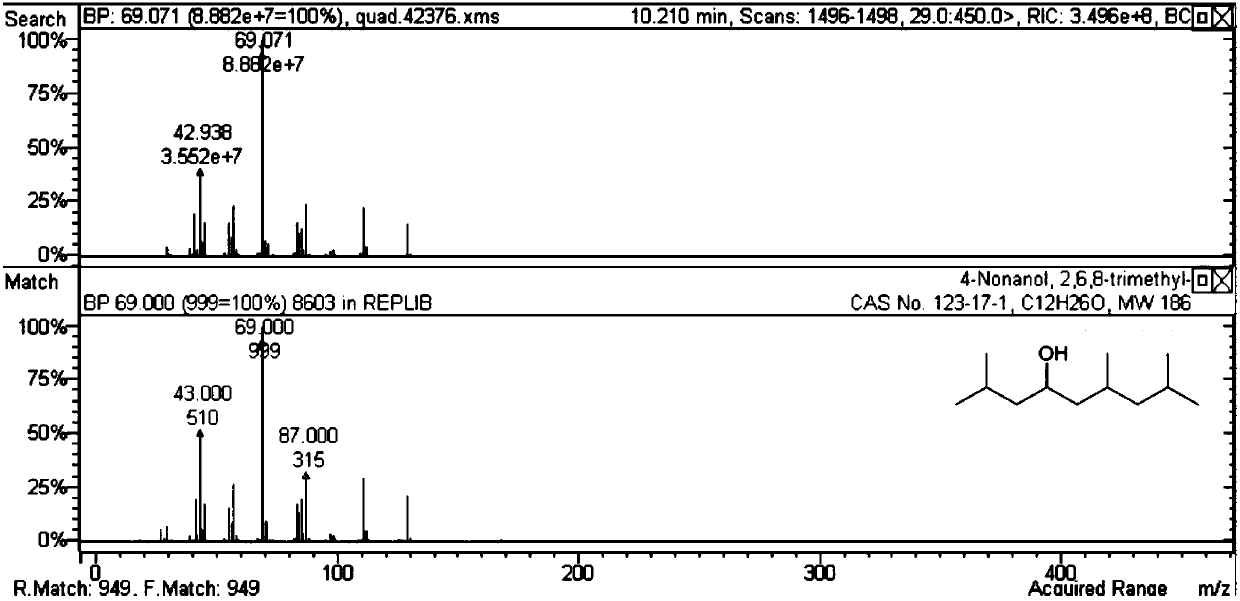
[0047] It can be seen from Table 2 that the self-polymerization reaction activity of methyl isobutyl ketone is not ideal for the solid acid and basic catalyst without metal doping. When doping noble metals on acid or base catalysts (Examples 19-35), carbon-numbered dodecanones and alcohols can be generated. Among them, the activity is better when the magnesium aluminum hydrotalcite doped with Pd.
[0048] Table 3 Self-condensation reaction target product structural formula
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com