Arylethyl piperidinyl derivatives and their application in the treatment of schizophrenia
A technology of arylethylpiperidine and derivatives, which is applied in the field of hydrate, salt or salt hydrate, and can solve problems such as cognitive impairment and poor curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
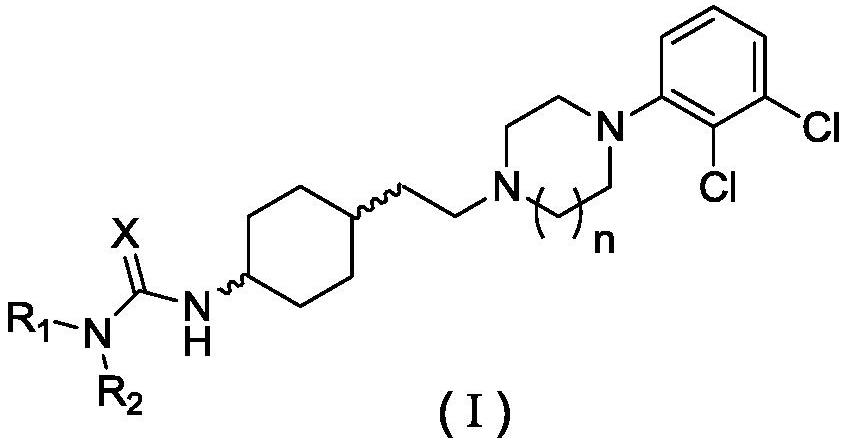
[0221] Embodiment 1: N-(4-(2-(4-(2,3-dichlorophenyl) piperazin-1-yl) ethyl) piperidin-1-yl) acetamide (compound Preparation of material 1-1) and salt thereof
[0222] 4-(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)ethyl)piperidin-1-amine (9) (prepared according to General Method 1) (1.0g, 2.8 mmol), triethylamine (4.2mmol) were added to dichloromethane (10mL), and a solution of acetyl chloride (0.24g, 3.1mmol) in dichloromethane (5mL) was added dropwise, and reacted for 3h, followed by water (20mL×2 ), washed with saturated brine (10 mL×2), dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a white solid, which was crystallized from 95% ethanol solution to obtain 1.1 g of a white solid with a yield of 95%.
[0223] 1 HNMR (CDCl 3 ,δ:ppm):1.19-1.36(m,2H,A-H),1.44-1.53(m,4H,A-H),1.71-1.74(m,1H,A-H),2.15(s,3H,A-H),2.29- 2.35(m,2H,A-H),2.40(t,2H,J=8.0Hz,N-CH 2 ),2.61(brs,4H,A-H),3.03(brs,4H,A-H),3.10-3.12(m,2H,A-H),6.92-6.95(m,1H,Ar-H),7.08-7.13(m, 2H,A...
Embodiment 2
[0238] Embodiment 2: N-(4-(2-(4-(2,3-dichlorophenyl) piperazin-1-yl) ethyl) piperidin-1-yl) butanamide (compound The preparation of thing 1-2) and salt thereof
[0239] Using intermediate 9 (2.8 mmol) and butyryl chloride (3.1 mmol) as raw materials, according to the preparation method of compound I-1, 1.14 g of the target compound I-2 was obtained as a white solid with a yield of 95%.
[0240] 1 HNMR (CDCl 3 ,δ:ppm):0.95(t,3H,J=6.8Hz,A-H),1.17-1.34(m,2H,A-H),1.43-1.52(m,4H,A-H),1.68-1.71(m,3H, A-H),2.28-2.34(m,2H,A-H),2.36(t,2H,J=7.2Hz,A-H),2.39(t,2H,J=8.0Hz,N-CH 2 ),2.59(brs,4H,A-H),3.01(brs,4H,A-H),3.09-3.10(m,2H,A-H),6.90-6.93(m,1H,Ar-H),7.06-7.12(m, 2H,Ar-H).
[0241] ESI-MS:427[M+H + ].
[0242] The preparation of compound 1-2 hydrobromic acid salt
[0243] Using compound I-2 (2.0 mmol) and 5% hydrobromic acid aqueous solution (2.1 mmol) as raw materials, the preparation method of compound I-1 hydrobromide was used to obtain 0.9 g of white solid with a yield o...
Embodiment 3
[0245] Example 3: N-(4-(2-(4-(2,3-dichlorophenyl)piperazin-1-yl)ethyl)piperidin-1-yl)-2-methoxyethyl Preparation of amides (compound I-3) and their salts
[0246] Using intermediate 9 (2.8 mmol) and 2-methoxyacetyl chloride (3.1 mmol) as raw materials, according to the preparation method of compound I-1, 1.1 g of the target compound I-3 was obtained as a white solid with a yield of 92%.
[0247] 1 HNMR (CDCl 3 ,δ:ppm):1.18-1.36(m,2H,A-H),1.44-1.70(m,5H,A-H),2.32-2.38(m,2H,A-H),2.43(t,2H,J=8.0Hz, N-CH 2 ),2.63(brs,4H,A-H),3.06(brs,4H,A-H),3.12-3.14(m,2H,A-H),3.32(s,3H,A-H),4.30(s,2H,A-H),6.93 -6.97(m,1H,Ar-H),7.10-7.16(m,2H,Ar-H).
[0248] ESI-MS:429[M+H + ].
[0249] Preparation of Compound I-3 Fumarate
[0250] Using compound I-3 (2.3 mmol) and fumaric acid (2.4 mmol) as raw materials, the preparation method of compound I-1 hydrobromide was used to obtain 1.0 g of white solid with a yield of 72%.
[0251] Elemental Analysis: C 20 h 30 Cl 2 N 4 O·C 4 h 4 o 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com