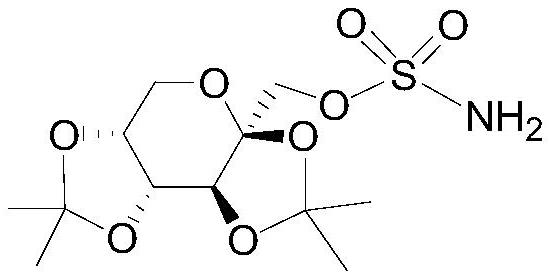
A kind of refining method of topiramate
A refining method and technology of topiramate, applied in chemical instruments and methods, preparation of sugar derivatives, organic chemistry, etc., can solve the problems that the purification technology cannot meet the requirements for the production of pharmaceutical grade topiramate, and fail to achieve simple impurities, so as to avoid multiple One-step purification operation, shortening production cycle, improving economy and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Get topiramate crude product, carry out refining according to the following steps:
[0036] Add 1kg of 2,3:4,5-bis-O-(1-methylethylene)-β-D-fructopyranose sulfamate oily liquid to 0.5L methanol and 0.5L ethanol, heat to reflux until dissolved , then lower the temperature to 55°C, adjust the pH to 9.0 with 20% ammonia water by mass fraction, and obtain system I;
[0037] Stir and keep warm at 50°C, add 10L of petroleum ether dropwise to System I, after 1.5h, the dropwise addition is completed, then cool down to room temperature naturally, and stand for crystallization for 8 hours to obtain System II;
[0038] The system II was filtered, and the filter cake was put into 1 L of petroleum ether for rapid stirring and grinding, filtered, and vacuum-dried to obtain pure topiramate crystals with a yield of 93.8%. The obtained pure topiramate crystals were detected by HPLC, and the results showed that the purity calculated by the external standard method was 99.86%, and the im...
Embodiment 2
[0040] Get topiramate crude product, carry out refining according to the following steps:
[0041] Add 1 kg of 2,3:4,5-bis-O-(1-methylethylidene)-β-D-fructopyranose sulfamate oily liquid to 4L propanol, heat to reflux until dissolved, then cool down to At 55°C, adjust the pH to 9.2 with 20% ammonia water to obtain System I;
[0042] Stir and keep warm at 52°C, add 5 L of ethylene glycol dimethyl ether dropwise to System I, after 1.2 hours, the dropwise addition is completed, cool down to room temperature naturally, and stand for crystallization for 9 hours to obtain System II;
[0043] The system II was filtered, and the filter cake was put into 3 L of ethylene glycol dimethyl ether for rapid stirring and grinding, filtered, and vacuum-dried to obtain pure topiramate crystals with a yield of 95.1%. The obtained pure topiramate crystals were detected by HPLC, and the results showed that the calculated purity by the external standard method was 99.85%, and the impurities with r...
Embodiment 3
[0045] Get topiramate crude product, carry out refining according to the following steps:
[0046] Add 1kg of 2,3:4,5-bis-O-(1-methylethylidene)-β-D-fructopyranose sulfamate oily liquid to 5L of ethanol, heat to reflux until dissolved, then cool down to 57 °C, adjust the pH to 9.5 with 25% ammonia water to obtain system I;
[0047] Stir and keep warm at 55°C, add 3 L of petroleum ether dropwise to System I, after 1.2 hours, the dropwise addition is completed, then cool down to room temperature naturally, and stand for crystallization for 10 hours to obtain System II;
[0048] The system II was filtered, and the filter cake was put into 5 L of petroleum ether, ethylene glycol dimethyl ether, and methyl tert-butyl ether, stirred and ground rapidly, filtered, and vacuum-dried to obtain pure topiramate crystals with a yield of 92.2%. The obtained pure topiramate crystals were detected by HPLC, and the results showed that the purity of topiramate calculated by the external standar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com