Synthesis process of pentaerythritol tetra(2-ethylhexanoate)
A technology of pentaerythritol tetra- and isooctanoate, which is applied in the separation/purification of carboxylic acid esters, the preparation of carboxylic acid esters, and the preparation of organic compounds, etc., and can solve the problems of non-compliance with green chemistry, difficulty in industrial production, unfavorable industrial production, etc. , to achieve the effect of good catalytic effect, sufficient reaction and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
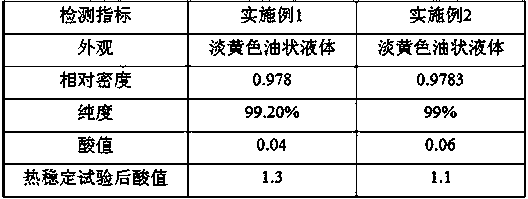
Embodiment 1
[0020] (1) Preparation of solid superacid catalyst.
[0021] Take 25g TiCl 4 Dissolve in water, then hydrolyze with dilute ammonia water to become alkaline (PH=11), let stand for 24 h, filter and wash to obtain a large amount of white precipitate. The solid was dried at 120 °C and ground into powder. Then add it to the concentrated sulfuric acid solution of 0.5 M cerium sulfate, react for 10 h, filter and dry, put it in the muffle furnace to roast, and obtain the superacid catalyst SO 4 2- / TiO 2 / Ce.
[0022] (2) Synthesis of pentaerythritol tetraisocaprylate.
[0023] Pentaerythritol (100 g) and isooctanoic acid (529 g) were added to the reaction vessel, and then the catalyst (10 g) prepared in the above step was added to the reaction vessel, the reaction temperature was 150 °C, and the reaction was carried out for 13 h. After the reaction was completed, the heating was stopped to obtain the crude product.
[0024] (3) Post-processing of products.
[0025] The reacti...
Embodiment 2
[0027] (1) Preparation of solid superacid catalyst.
[0028] Take 50g TiCl 4 Dissolve in water, then hydrolyze with dilute ammonia water to become alkaline (PH=10), let it stand for 24 hours, filter and wash, and get a large amount of white precipitate. The solid was dried at 110 °C and ground into powder. Then add it to the concentrated sulfuric acid solution of 0.5 M cerium sulfate, react for 11 h, filter and dry, put it in a muffle furnace and roast it to obtain the superacid catalyst SO 4 2- / TiO 2 / Ce.
[0029] (2) Synthesis of pentaerythritol tetraisocaprylate.
[0030] Add pentaerythritol (200 g) and isooctanoic acid (1.19 g) into the reaction vessel, then add the catalyst (15 g) prepared in the above step to the reaction vessel, the reaction temperature is 120 ° C, react for 11 h, stop heating after the reaction to obtain the crude product .
[0031] (3) Post-processing of products.
[0032] The reaction solution is filtered, and the catalyst is filtered out a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com