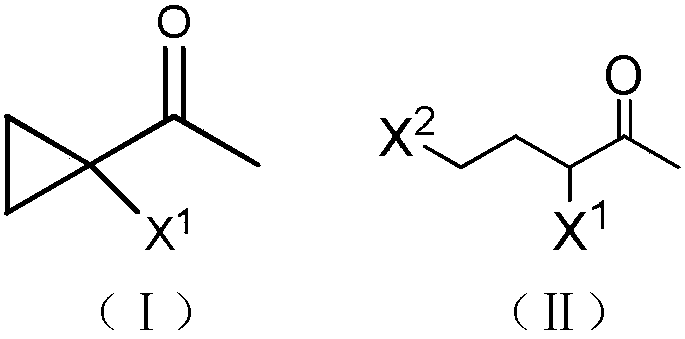
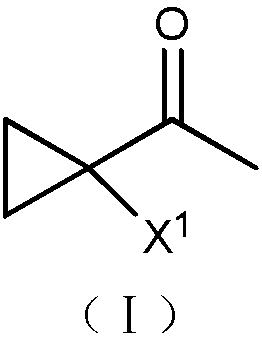
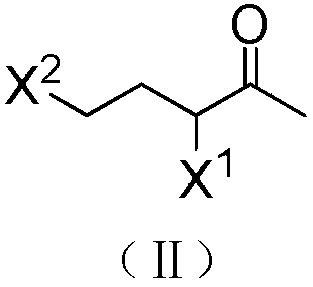
Synthetic method of 1-halo-1-acetyl cyclopropane
A technology based on cyclopropane and a synthesis method, which is applied in the field of synthesis of pharmaceutical and pesticide intermediates, can solve the problems of harsh reaction conditions, complicated operation, long reaction time, etc., and achieves a short time consumption, harsh reaction conditions, and mild conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] At 0°C, 50 mL of dichloromethane, 6 g (0.15 mol) of sodium hydroxide and 0.09 g (4×10 -4 mol) benzyltriethylammonium chloride, and then 15.5 grams (0.1 mol) of 3,5-dichloro-2-pentanone was added dropwise. After the dropwise addition, the temperature was raised, refluxed for 0.5 hours, and cooled to room temperature. The reaction solution was washed twice with water, separated, and the organic phase was dried over anhydrous sodium sulfate and evaporated to dryness to obtain 12 g of a light yellow transparent liquid. It was confirmed to be 1-chloro-1-acetylcyclopropane by NMR analysis, with a yield of 96.5% and a purity of 95.3%. 1 H NMR (CDCl 3 ,500MHz, δ / ppm):2.46(s,3H),1.64(dd,J=8.5,5.0Hz,2H),1.35(dd,J=8.5,5.0Hz,2H).
Embodiment 2
[0017] At 0°C, 50 mL of dichloromethane, 6 g (0.15 mol) of sodium hydroxide and 0.09 g (4×10 -4 mol) benzyltriethylammonium chloride, and then 20 g (0.1 mol) of 3-chloro-5-bromo-2-pentanone was added dropwise. After the dropwise addition, the temperature was raised, refluxed for 0.5 hours, and then cooled to room temperature. The reaction solution was washed twice with water and separated. The organic phase was dried over anhydrous sodium sulfate and evaporated to dryness to obtain 11.9 g of a light yellow transparent liquid. It was confirmed to be 1-chloro-1-acetylcyclopropane by NMR analysis, with a yield of 98% and a purity of 97.5%.
Embodiment 3
[0019] At 0°C, 50 mL of toluene, 8 g (0.2 mol) of sodium hydroxide and 0.09 g (4×10 - 4 mol) benzyltriethylammonium chloride, and then 20 g (0.1 mol) of 3-bromo-5-chloro-2-pentanone was added dropwise. After the dropwise addition, the temperature was raised to 100° C., reacted for 2 hours, and then cooled to room temperature. The reaction solution was washed twice with water and separated. The organic phase was dried over anhydrous sodium sulfate and evaporated to dryness to obtain 15.9 g of a light yellow transparent liquid. It was confirmed to be 1-bromo-1-acetylcyclopropane by NMR analysis, with a yield of 84% and a purity of 86%. 1 HNMR (CDCl 3 ,500MHz,δ / ppm):2.47(s,3H),1.68-1.71(m,2H),1.41-1.44(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com