Series of open-loop abietane diterpene compounds, and pharmaceutical compositions and application thereof in pharmacy
A kind of compound, technology of rosinane, applied in a series of ring-opening rosinane type diterpenoids and their pharmaceutical compositions and their application fields in pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
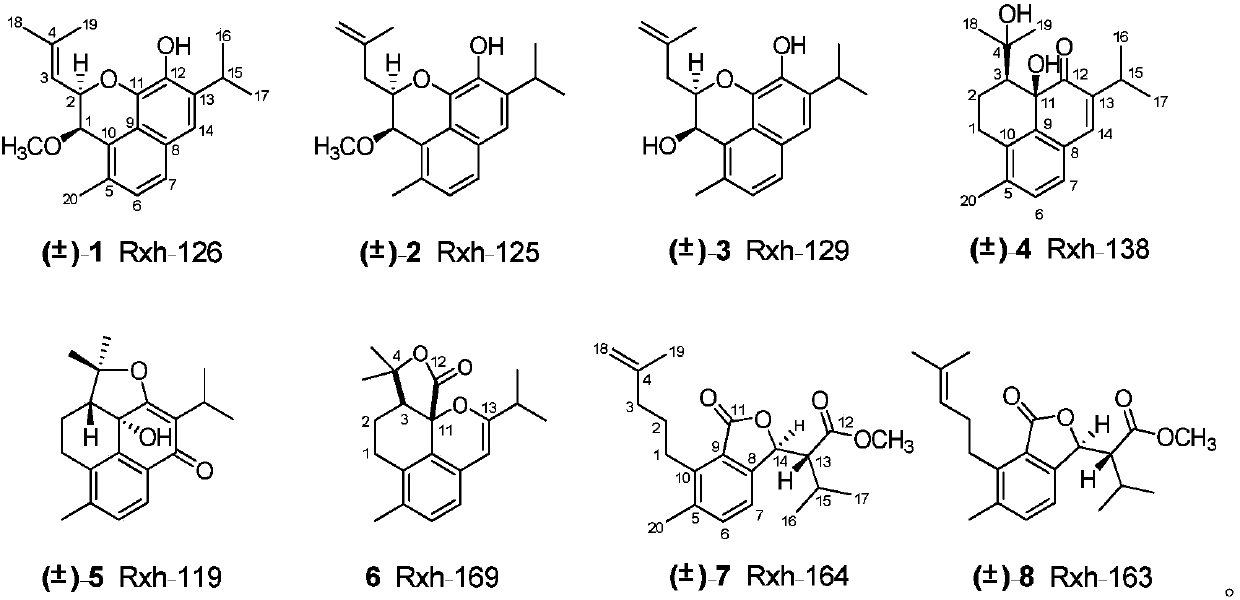
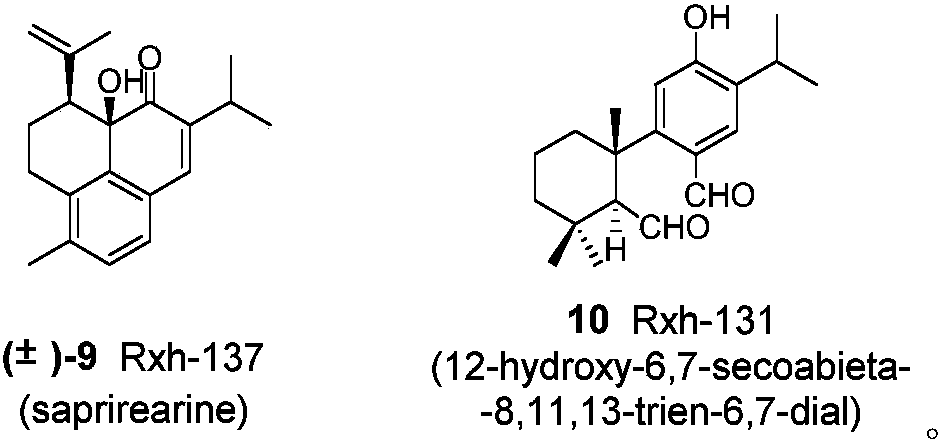
[0021] Compounds Rxh-126(1), Rxh-125(2), Rxh-129(3), Rxh-138(4), Rxh-119(5), Rxh-169(6), Rxh-164(7), Preparation and structural data of Rxh-163(8), Rxh-137(9), Rxh-131(10):
[0022] The whole herb of Sage Kangding is 38kg, crushed and cold-soaked with acetone at room temperature for 3 times (48 hours / time), combined the extracts, and distilled under reduced pressure to obtain the extract. It was dissolved in silica gel to mix the sample, and the silica gel column chromatography was eluted with chloroform, ethyl acetate, and methanol respectively to obtain a chloroform fraction (667 g). The chloroform part was mixed with silica gel, silica gel column chromatography, and mixed with petroleum ether / chloroform / ethyl acetate (50:1:1, 20:1:1, 10:1:1, 5:1:1, 1:1: 1) Elution yields 7 sections A-G. Among them, section B (223g) was mixed with polyamide, passed through the MCI column, and carried out gradient elution with methanol-water (70:30-100:0) as the mobile phase to obtain 8 sec...
Embodiment 2
[0052] Effects of compounds 1-10 on isolated rat thoracic aorta:
[0053] 1. Preparation of vascular rings: Take out the rat thoracic aorta quickly, carefully remove the surrounding connective tissue of the blood vessel wall, and cut the blood vessels into 3-4mm vascular rings. Transfer the vascular ring to a bath filled with 5mL krebs solution, kept at a constant temperature of 37°, and continuously pass mixed oxygen, and fix it on a hook in the bath, and connect the other hook to a tension transducer, and record it with the RM-6240 system Tension of vascular rings. Before the experiment, the blood vessels were given a basic tension of 1.5g in advance, and the blood vessels were balanced for 60 minutes, and the liquid was changed every 15 minutes during this period. After the balance of the vascular ring is stable, use phenylephrine 10 -5 mol / L pre-constricted vascular ring reaches the peak value, add acetylcholine 10 -5 mol / L to verify the integrity of the endothelium. If...
Embodiment 3
[0059] Preparation of tablets:
[0060] Obtain ring-opening abietane type diterpenoids 1-10 by the method for embodiment 1-5, and utilize organic acid (tartaric acid, citric acid, formic acid, oxalic acid etc.) or mineral acid (hydrochloric acid, sulfuric acid, phosphoric acid etc.) ) made salt, adding excipients in the ratio of 1:5-1:10 by weight to the excipients, granulating and compressing into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com