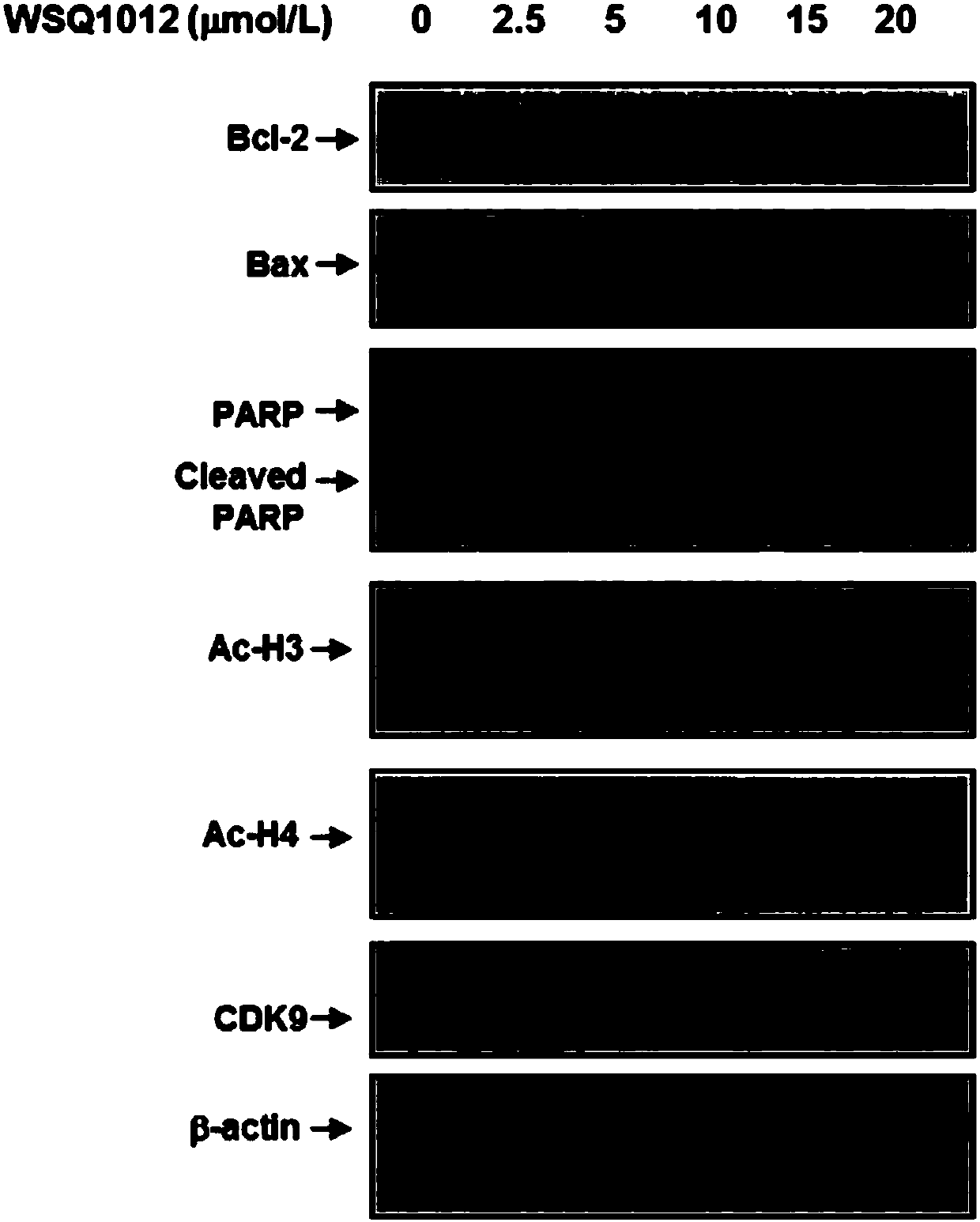
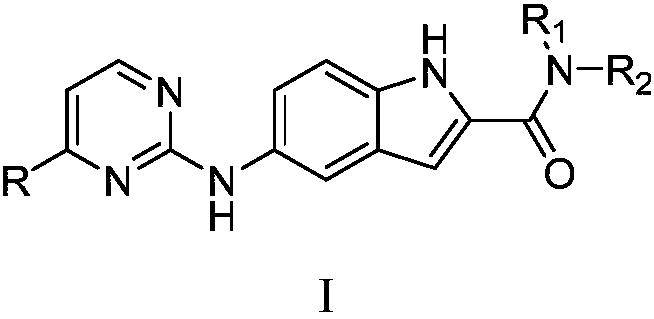
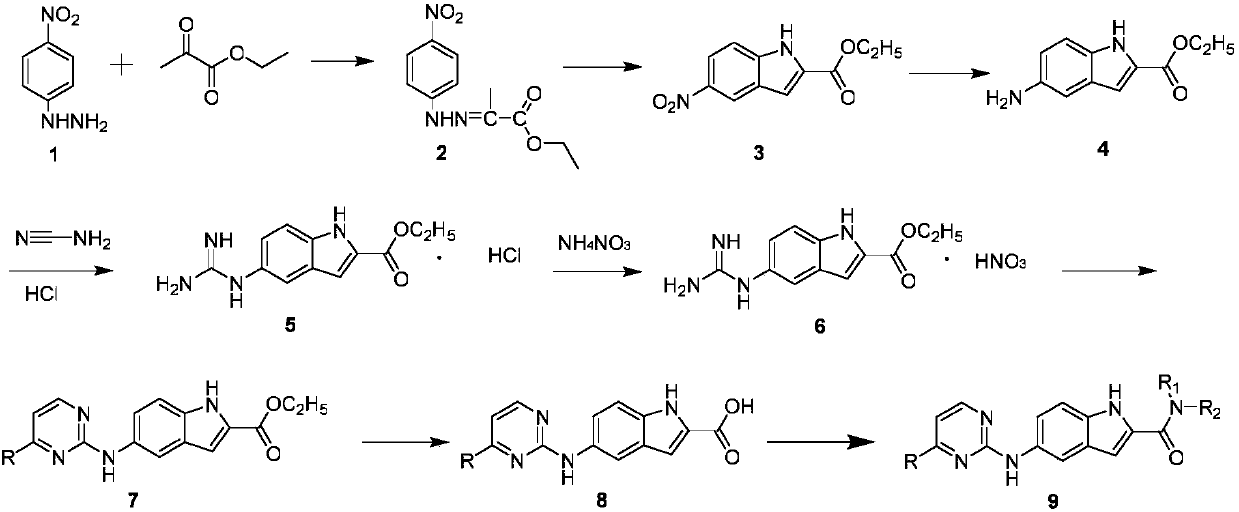
N-substituted-5-((4-substituted pyrimidine-2-yl)amino)indole derivative as well as preparation method and purpose thereof
A technology for indole derivatives and derivatives, applied in the field of N-substituted-5-amino) indole derivatives and their preparation, capable of solving problems such as toxic and side effects, achieving simple preparation methods, low reaction costs, and simple reaction processes easy to control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of N-butyl-5-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)-1H-indole-2-carboxamide
[0037] Preparation of ethyl 5-aminoindole-2-carboxylate
[0038]
[0039] Add p-nitrophenylhydrazine (10g, 0.065mol) and absolute ethanol (85mL) sequentially into a 250mL dry two-necked bottle, then add ethyl pyruvate (8.14g, 0.072mol) dropwise under stirring, and the addition ends Afterwards, the temperature was raised and heated to reflux, and after 2 hours of reflux reaction, TLC detected that the reaction was over, and the reaction was stopped. The reaction solution was cooled to room temperature, filtered with suction, and the filter cake was collected and dried to obtain 13.8 g of ethyl pyruvate p-nitrophenylhydrazone as a yellow solid product, with a yield of 89.6% and a melting point (m.p.): 197-199°C.
[0040] In a dry 500mL reaction flask, first add ethyl pyruvate p-nitrophenylhydrazone (10g, 0.042mol) and polyphosphoric acid (110g) in sequence, then raise...
Embodiment 2
[0049] Example 2: N-(3-(diethylamino)propyl)-5-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)-1H-indole-2-carboxamide preparation of
[0050] The reaction procedure was the same as that in Example 1, except that n-butylamine was replaced by 3-diethylaminopropylamine (39 mg, 0.3 mmol), and finally 67.18 mg of a pale yellow solid product was obtained with a yield of 26.6%. Spectral data: 1 H NMR (600MHz, DMSO-d6): δ11.45 (d, J = 0.9Hz, 1H), 9.56 (s, 1H), 9.35 (d, J = 1.6Hz, 1H), 8.73 (dd, J = 1.6 ,4.8Hz,1H),8.57(d,J=4.9Hz,1H),8.49(td,J=1.9,8.0Hz,1H),8.47(t,J=5.5Hz,1H),8.13(s,1H ),7.58(ddd,J=0.6,4.8,7.9Hz,1H),7.48(dd,J=2.0,8.8Hz,1H),7.43(d,J=5.1Hz,1H),7.37(d,J= 8.8Hz, 1H), 7.04(d, J=1.4Hz, 1H), 3.28~3.33(m, 2H), 2.46(quin, J=7.4Hz, 6H), 1.67(quin, J=7.0Hz, 2H) ,0.96(t,J=7.1Hz,6H); 13 C NMR (151MHz, DMSO-d6): δ161.9, 161.4, 161.1, 159.8, 151.9, 148.6, 134.8, 133.4, 133.2, 132.9, 132.8, 127.5, 124.4, 118.8, 112.5, 111.6, 107.8, 102.4, 5( *2),38.0,27.2,12.2(*2);ESI-MS(+):[M+H] + ,44...
Embodiment 3
[0051] Example 3: Preparation of N-benzyl-5-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)-1H-indole-2-carboxamide (WSQ-1026)
[0052] The reaction procedure was the same as that in Example 1, except that n-butylamine was replaced by benzylamine (32.01 mg, 0.3 mmol), and finally 56.8 mg of a light yellow solid product was obtained by isolation, with a yield of 43%. Spectral data: 1H NMR (600MHz, DMSO-d6): δ11.52(brs, 1H), 9.58(br s, 1H), 9.36(d, J=2.0Hz, 1H), 9.00(t, J=6.0Hz, 1H) ,8.73(dd,J=1.6,4.7Hz,1H),8.57(d,J=5.1Hz,1H),8.49(td,J=1.9,8.0Hz,1H),8.15(s,1H),7.59( dd,J=4.6,7.9Hz,1H),7.50(dd,J=1.9,8.8Hz,1H),7.43(d,J=5.1Hz,1H),7.39(d,J=8.8Hz,1H), 7.34~7.37(m,4H),7.26(dt,J=2.7,5.8Hz,1H),7.16(d,J=1.4Hz,1H),4.53(d,J=6.0Hz,2H); 13 C NMR (151 MHz, DMSO-d6): δ161.9, 161.6, 161.1, 159.8, 151.9, 148.6, 140.1, 134.8, 133.5, 133.3, 132.9, 132.4, 128.8, 127.7, 127.5, 127.3, 126.4, 11129.5, ,107.8,103.0,42.6; ESI-MS(+):[M+H] + ,421.17.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com