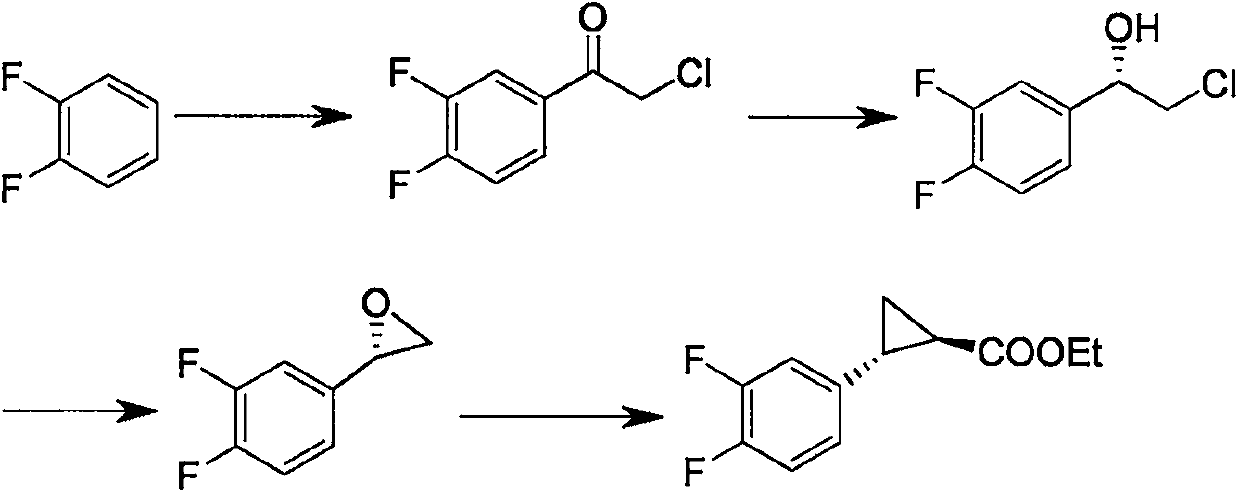
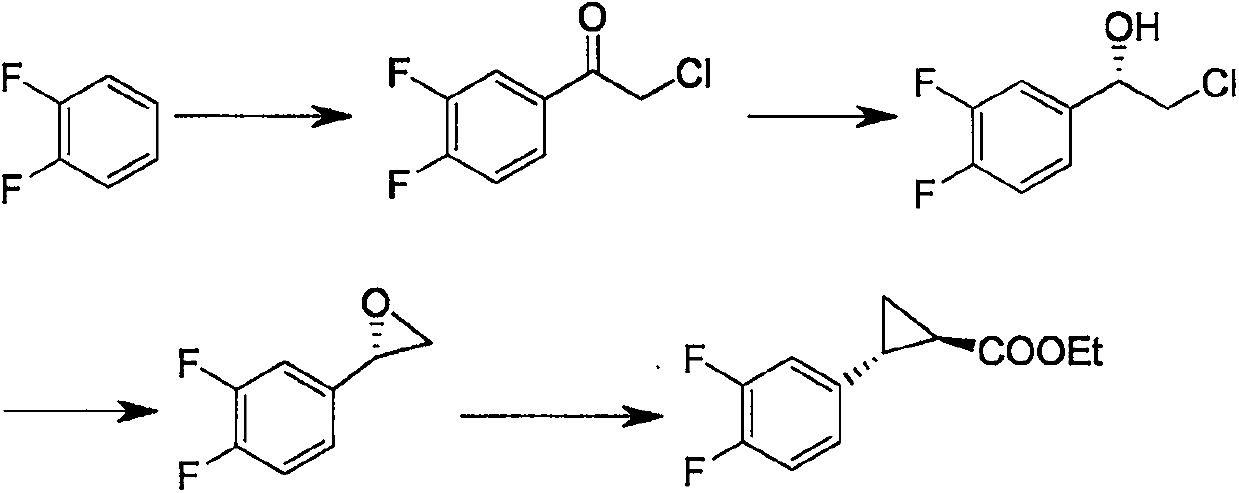
Method for preparing ticagrelor intermediate
A technology for ticagrelor and intermediates, which is applied in the field of synthesis of chemical drug intermediates, can solve the problems of high cost, unfavorable industrialization, and high risk of difluorobenzaldehyde, and achieve fewer staff, stable product quality, and high selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Put (114.1g, 1.0mol) o-difluorobenzene, (146.7g, 1.1mol) aluminum trichloride in a 1L three-neck flask, stir, cool down to 15°C, and dropwise add (113.0g, 1.0mol) chloroacetyl chloride Control the temperature not to exceed 40°C, keep the reaction at 40°C for 2 hours, cool down to 5°C, drop the reaction liquid into 10% hydrochloric acid, control the temperature at 20°C, extract with 228g of dichloromethane, distill off the dichloromethane to obtain 178.5g 2-Chloro-1-(3,4-difluorophenyl)ethanone, yield 93.7%.
[0022] Add (178.5g, 0.94mol) 2-chloro-1-(3,4-difluorophenyl)ethanone, 250g glucose, and 900g water into a 2L three-necked flask, stir and mix, and adjust the pH to 6.5, temperature 20°C, add 35.7g Candida parapsilosis enzyme, keep pH 6.5, temperature 20°C, react for 3 hours, add 357.0g toluene for extraction, and separate liquid to obtain (S)-2-chloro-1-( 3,4-difluorophenyl) ethane-1-ol toluene solution;
[0023] Add (S)-2-chloro-1-(3,4-difluorophenyl)ethan-1-ol ...
Embodiment 2
[0026] Put (114.1g, 1.0mol) o-difluorobenzene, (133.4g, 1.0mol) aluminum trichloride in a 1L three-neck flask, stir, cool down to 25°C, and dropwise add (124.3g, 1.1mol) chloroacetyl chloride Control the temperature not to exceed 40°C, keep the reaction at 35°C for 4 hours, cool down to 8°C, drop the reaction liquid into 10% hydrochloric acid, control the temperature at 20°C, extract with 228g of dichloromethane, distill off the dichloromethane to obtain 175.6g 2-Chloro-1-(3,4-difluorophenyl)ethanone, yield 92.2%.
[0027] Add (175.6g, 0.92mol) 2-chloro-1-(3,4-difluorophenyl)ethanone, 300g glucose, and 900g water into a 2L three-necked flask, stir and mix, and adjust the pH to 7.5, temperature 30°C, add 52.7g Candida parapsilosis enzyme, keep pH 7.5, temperature 30°C, react for 5 hours, add 351.2g toluene for extraction, and separate liquid to obtain (S)-2-chloro-1-( 3,4-difluorophenyl) ethane-1-ol toluene solution;
[0028] Add (S)-2-chloro-1-(3,4-difluorophenyl)ethan-1-ol ...
Embodiment 3
[0031] Put (114.1g, 1.0mol) o-difluorobenzene, (146.7g, 1.1mol) aluminum trichloride in a 1L three-necked flask, stir, cool down to 15°C, and dropwise add (124.3g, 1.1mol) chloroacetyl chloride Control the temperature not to exceed 40°C, keep the reaction at 35°C for 5 hours, lower the temperature to 5°C, drop the reaction liquid into 10% hydrochloric acid, control the temperature at 15°C, extract with 228g of dichloromethane, distill off the dichloromethane to obtain 182.1g 2-Chloro-1-(3,4-difluorophenyl)ethanone, yield 95.6%.
[0032] Add (182.1g, 0.96mol) 2-chloro-1-(3,4-difluorophenyl)ethanone, 350g glucose, and 900g water into a 2L three-necked flask, stir and mix, and adjust the pH to 7.0, temperature 30°C, add 35.7g Candida parapsilosis enzyme, keep pH 7.0, temperature 30°C, react for 6 hours, add 364g toluene for extraction, and separate liquid to obtain (S)-2-chloro-1-(3 , 4-difluorophenyl) ethane-1-ol toluene solution;
[0033] Add (S)-2-chloro-1-(3,4-difluoropheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com