Method for synthesizing 1,2,4-butanetriol by enzymatic reaction
A technology of enzymatic reaction and butanetriol, which is applied in the field of bioengineering, can solve the problems of low conversion rate and high production cost, and achieve the effects of improving capacity, avoiding many by-products, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
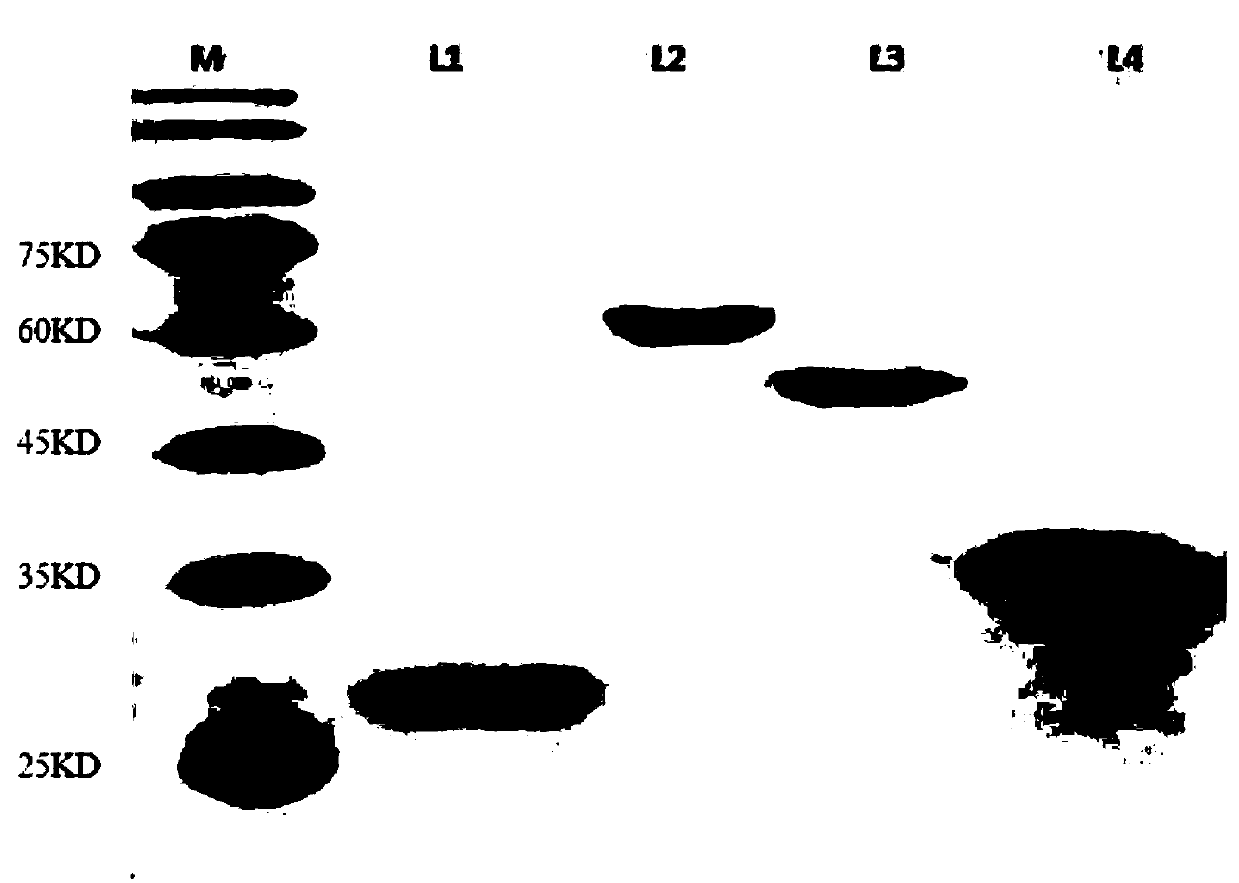
[0035] The preparation of pure enzyme solution related to in vitro synthesis of 1,2,4-butanetriol, the specific steps are as follows:
[0036] (1) Introduce restriction sites BamHI and HindIII at the 5' end and 3' end of the D-xylose dehydrogenase xylB gene, respectively, perform double enzyme digestion on the xylB gene and PRSFDuet-1, and then connect the xylB gene to PRSFDuet- 1 on the carrier;
[0037] (2) at the 5' end and 3 end of the D-xylonate dehydratase xylD gene ’ Introduce restriction sites BamHI and XhoI into the end primers, perform double enzyme digestion on the xylD gene and PRSFDuet-1, and then connect the xylD gene to the PRSFDuet-1 vector;
[0038] (3) 5 in the benzoylformate decarboxylase mdlC gene ’ end and 3 ’ Restriction sites BamHI and SacI were introduced at the end; double restriction digestion was performed on the mdlC gene and PRSFDuet-1, and then the mdlC gene was connected to the PRSFDuet-1 vector;
[0039] (4) 5 in alcohol dehydrogenase adhP g...
Embodiment 2
[0043] D-1,2,4-butanetriol was synthesized in vitro, the specific steps are as follows:
[0044] Reaction system: the concentration of D-xylose dehydrogenase is 350U / mL, the concentration of D-xylose dehydratase is 250U / mL, the concentration of benzoylformate decarboxylase is 200U / mL, alcohol dehydrogenase The concentration is 300U / mL.
[0045] PBS (NaHPO 4 , NaH 2 PO 4 )50mM pH7.0, xylose 20g / L, MgCl 2 6mM, NAD + 0.5 mM, NADH 0.5mM, TPP 0.4mM; finally add PBS to 10mL, the reaction temperature is 37°C, the reaction time is 24h, and the product D-1,2,4-butanetriol is obtained.
[0046] According to the in vitro synthesis system of Example 2, 1,2,4-butanetriol was detected by high performance liquid chromatography, and the final yield was 12 g / L, and the yield was 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com