A method for detecting related substances of velpatasvir
A technology of related substances and detection methods, which is applied in the field of detection of velpatasvir related substances, can solve problems such as methods for the determination of velpatasvir related substances that have not been seen, and achieve good resolution, effective separation, and good reproducibility sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The test solution: take an appropriate amount of velpatasvir raw drug powder, dissolve it with methanol and quantitatively dilute it to a solution of 1.0 mg / mL;
[0037] Mobile phase A: 0.1% phosphoric acid aqueous solution;
[0038] Mobile phase B: acetonitrile;
[0039] Chromatographic column: 250mm×4.6mm, 5μm;
[0040] Filler: C 18 Alkylsilane bonded silica gel;
[0041] Column brand: Kromstar
[0042] Column temperature: 30°C;
[0043] Detection wavelength: 295nm;
[0044] Flow rate: 1.2mL / min;
[0045] Elution method: gradient elution;
[0046] Gradient elution conditions:
[0047]
[0048] Take 20 μl of the above-mentioned test solution, inject it into a high-performance liquid chromatograph, and record the chromatogram.
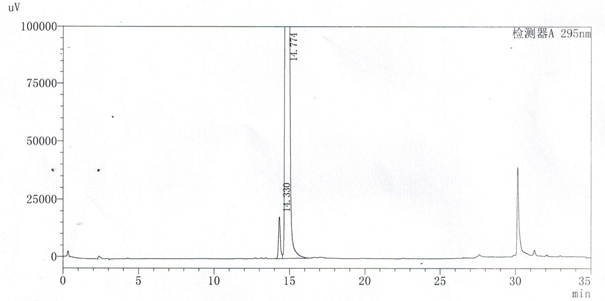
[0049] see results figure 1 , figure 1 The chromatographic peak at 14.330min is the chromatographic peak of velpatasvir related substance A, and the chromatographic peak at 14.774min is the chromatographic peak of velpatasvir. The...
Embodiment 2
[0051] The test solution: take an appropriate amount of velpatasvir raw drug powder, dissolve it with methanol and quantitatively dilute it to a solution of 1.0 mg / mL;
[0052] Mobile phase A: 0.1% phosphoric acid aqueous solution;
[0053] Mobile phase B: acetonitrile;
[0054] Chromatographic column: 250mm×4.6mm, 5μm;
[0055] Filler: C 18 Alkylsilane bonded silica gel;
[0056] Column brand: Agilent
[0057] Column temperature: 30°C;
[0058] Detection wavelength: 295nm;
[0059] Flow rate: 1.2mL / min;
[0060] Elution method: Gradient elution
[0061] Gradient elution conditions:
[0062]
[0063] Take 20 μl of the above-mentioned test solution, inject it into a high-performance liquid chromatograph, and record the chromatogram.
[0064] see results figure 2 , figure 2 The chromatographic peak at 12.450min is the chromatographic peak of velpatasvir related substance A, and the chromatographic peak at 12.925min is the chromatographic peak of velpatasvir. The...
Embodiment 3
[0065] Embodiment 3 Comparative Examples.
[0066] Take the sample in Example 1, and replace the mobile phase A in Example 1 with 0.1% trifluoroacetic acid aqueous solution for detection, and other chromatographic parameters are the same as in Example 1. The result is as image 3 As shown, and try to investigate different gradient elution conditions under this system, the results can not separate velpatasvir and its related substance A, showing that the main peak can not be separated from the main peak with the organic solvent acetonitrile as the mobile phase with trifluoroacetic acid aqueous solution and organic solvent acetonitrile The impurities are effectively separated, which is not suitable for the detection of related substances of velpatasvir.
[0067] In summary, the method for related substances of velpatasvir described in the present invention can be applied to the control of related substances of velpatasvir.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com