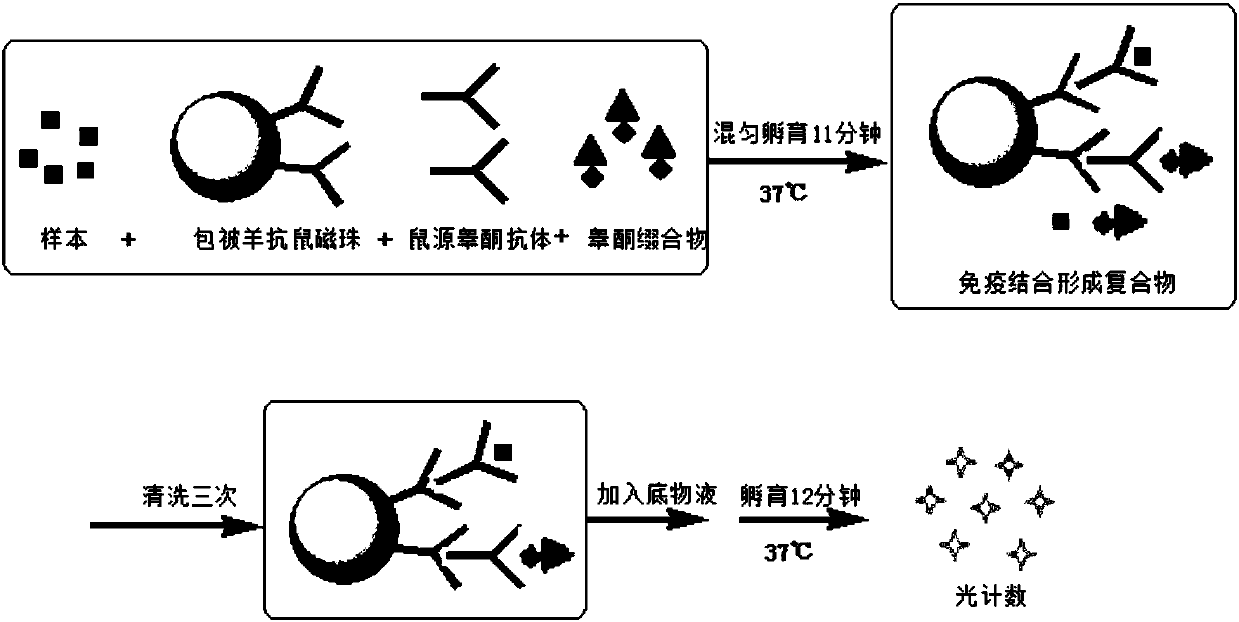
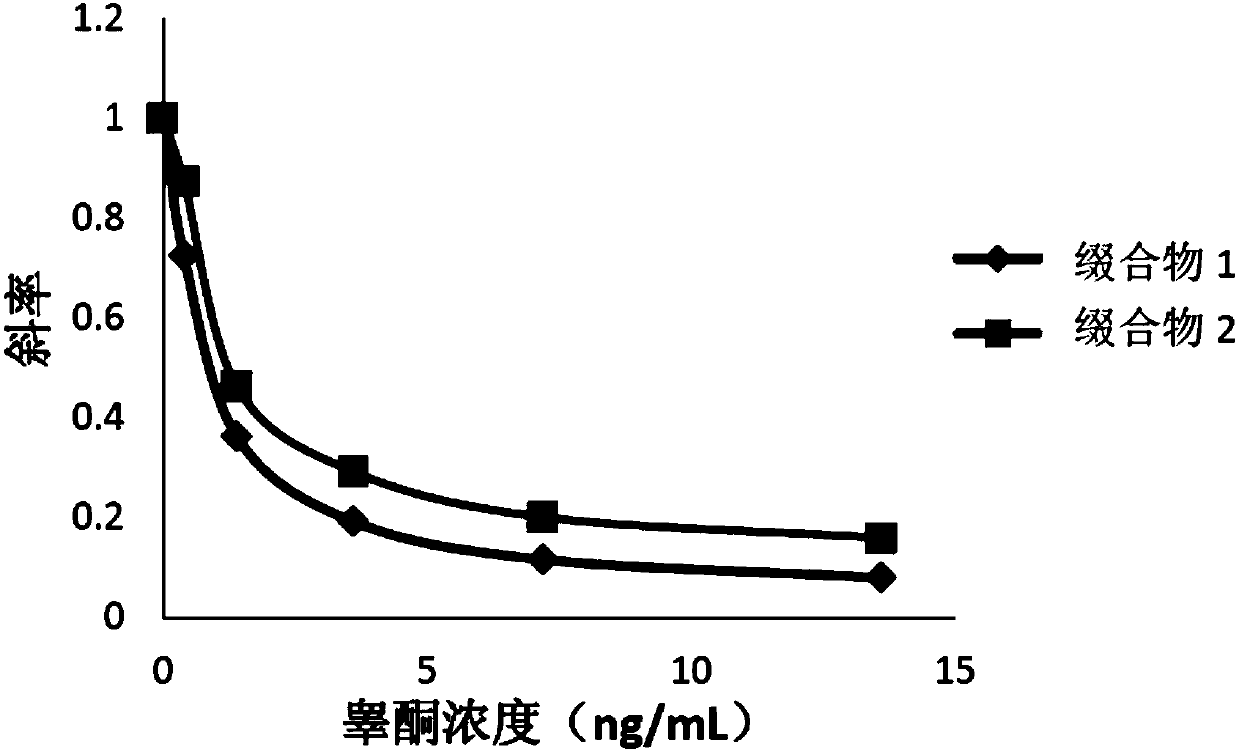
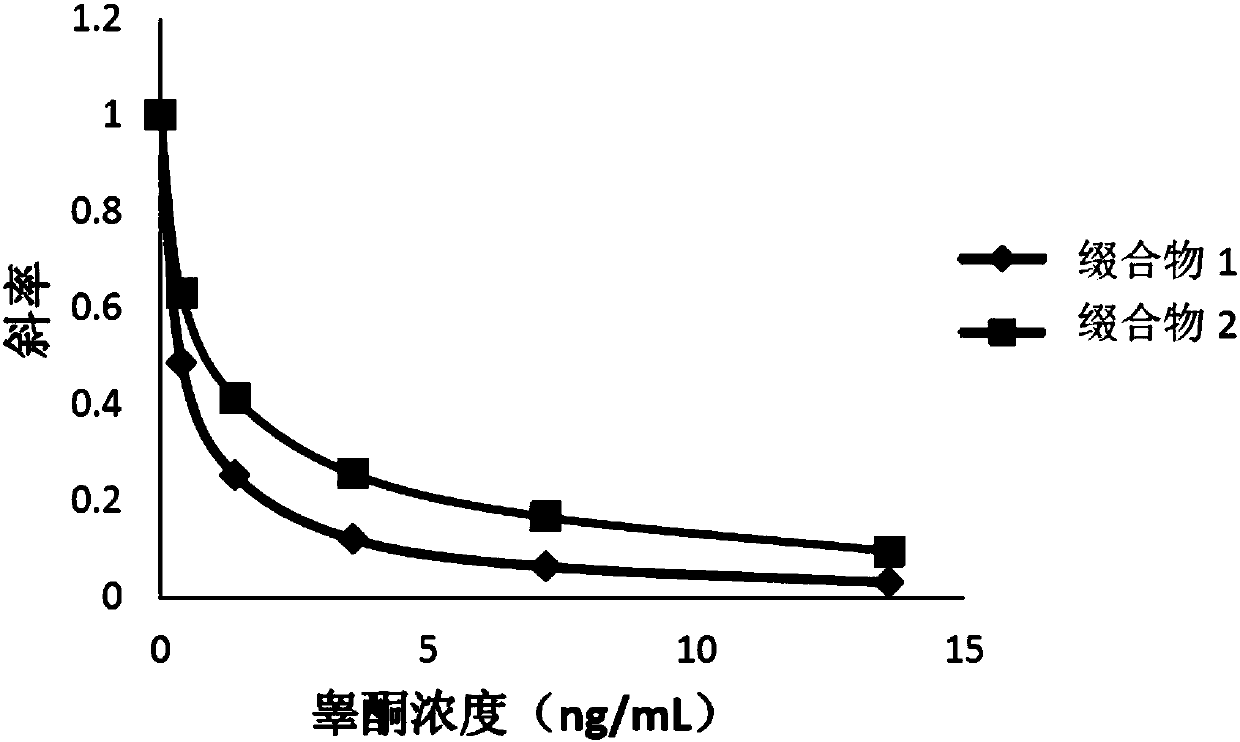
Compounds, conjugates, kits as well as applications thereof in detecting testosterone or analogues thereof
A technology of compounds and conjugates, applied in the field of analysis, can solve the problems of testosterone derivatives to be developed, and achieve the effect of being easy to popularize, apply and obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In this embodiment, the compound shown in formula (2) is synthesized according to the following process:
[0055]
[0056] The synthetic route is as follows:
[0057]
[0058] 1. Synthesis of compound 2:
[0059] Feed 3.05g (0.01mol) of compound 1, 2.8g (0.02mol) of anhydrous potassium carbonate and 0.5g of DMAP into a 100mL dry round bottom flask, add 50mL of DMF and stir to dissolve. Add 3.53 g (0.015 mol) of TBS-Cl (tri-n-butylchlorosilane), raise the temperature to 60° C., and react for 18 hours. Sampling was carried out regularly, and the sample was detected by thin-layer chromatography (the system was n-hexane / ethyl acetate=6 / 1, color developed by phosphomolybdic acid). When the raw materials disappeared, the heating was stopped. After returning to room temperature, the reaction solution was transferred to a 250 mL separatory funnel, 150 mL of ethyl acetate was added, washed 4 times with water, dried over anhydrous sodium sulfate, concentrated, and drained...
Embodiment 2
[0068] In this embodiment, the compound shown in formula (3) is synthesized according to the following process:
[0069]
[0070] The synthetic route is as follows:
[0071]
[0072] Compound shown in synthetic formula (3):
[0073] Feed 0.91g (3mmol) of compound 5 into a 50mL dry and cooled reaction flask, add 20mL of dichloromethane, stir to dissolve, add 0.2g of DMAP, 0.48g (4.8mmol) of succinic anhydride, and stir at room temperature overnight (16 hours). The reaction solution was transferred to a separatory funnel, washed with water four times, dried over anhydrous sodium sulfate, concentrated, and purified through a column to obtain 0.95 g of the compound represented by formula (3), with a reaction yield of 78.7%.
[0074] MS: positive ion peaks: 403.6, 425.6 (+Na peak); negative ion peaks: 401.5.
Embodiment 3
[0076] The compounds obtained in Examples 1 and 2 were labeled with alkaline phosphatase according to the following steps to obtain conjugates 1 and 2, respectively.
[0077] Add the compound to be labeled to Mes (2-(N-morpholine)ethanesulfonic acid) buffer solution (pH=4.5), the final concentration is 0.2mg / mL; add alkaline phosphatase (ALP), the final concentration is 0.2mg / mL, mix well; then add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDAC), the final concentration is 2mg / mL, mix well; add nitrogen hydroxysuccinyl Imine (NHS), with a final concentration of 2 mg / mL, mixed; reacted at 25°C for 2 hours; replaced by ultrafiltration three times with Tris buffer at pH = 7.5, purified to remove excess testosterone derivatives and cross-linking agents, to obtain the conjugate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com