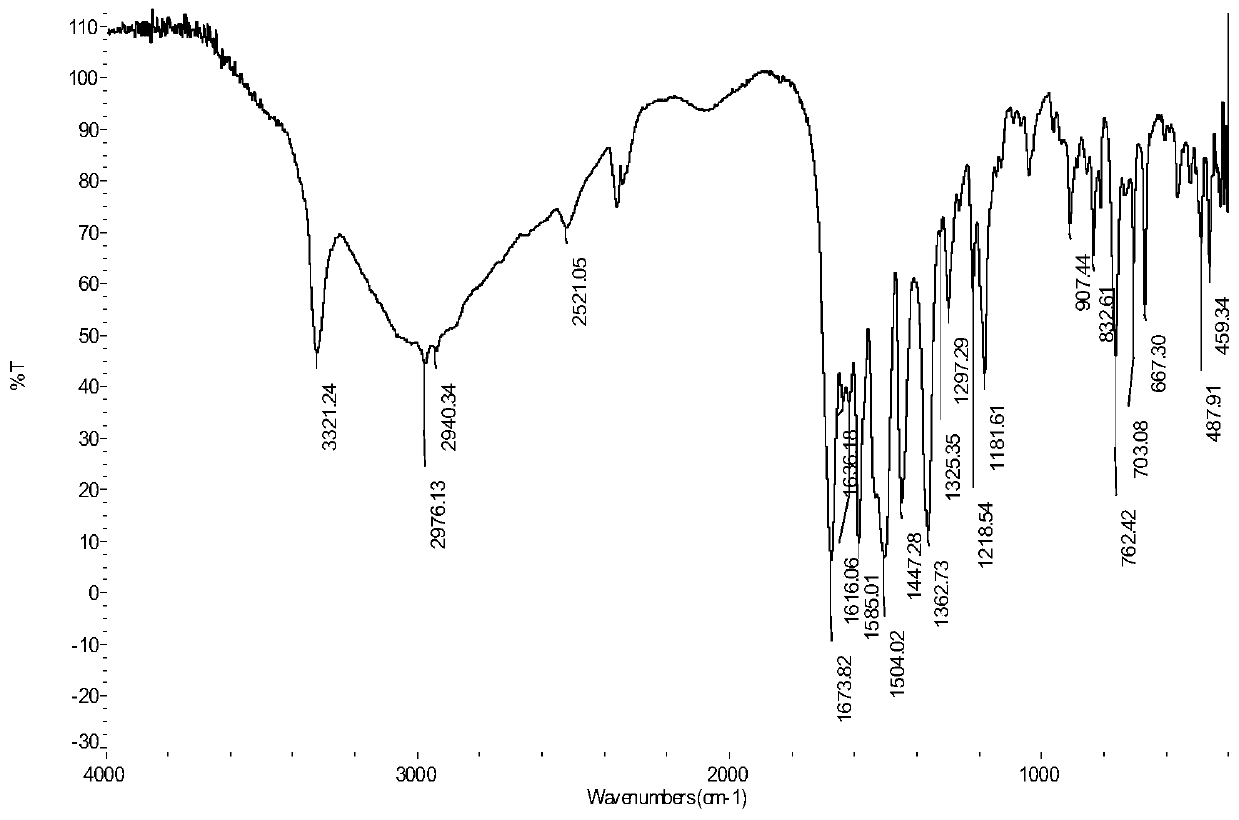
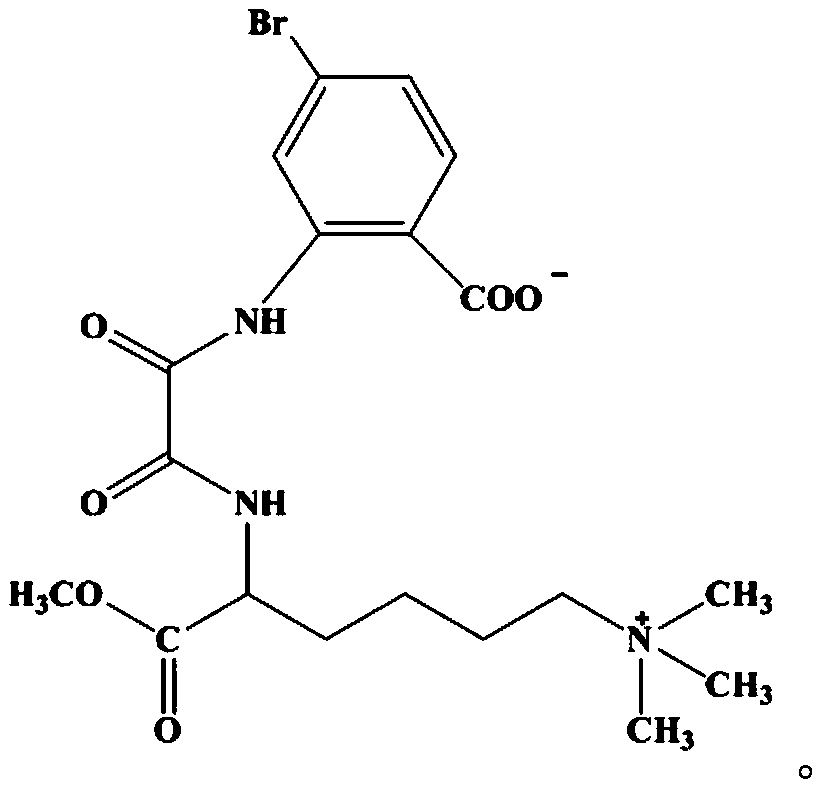
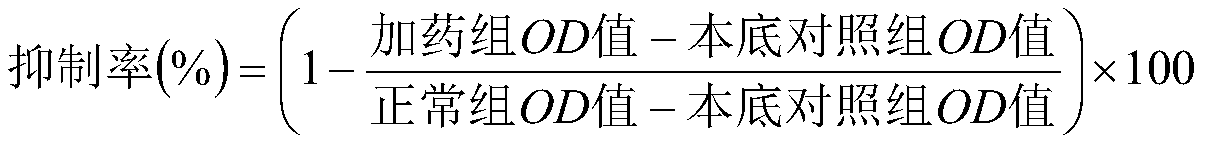Bromolaminarin-containing oxamide with anticancer activity and its synthesis method and application
A technology of lamininate and oxalamide, which is applied in the field of bromine-containing laminadinate and its synthesis, can solve the problems of NIK-3T3 toxicity and other problems, and achieve the goal of promoting apoptosis, precise treatment and better effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Synthesis of N-(5-bromo-2-carboxyanilino) oxalyl ethyl ester: Dilute 40mmol (5.49g) ethyl oxalate monoacyl chloride with 10mL of anhydrous tetrahydrofuran (THF) and place in an ice bath Slowly added dropwise to 40 mL of anhydrous THF solution dissolved with 4-bromo-2-aminobenzoic acid equimolar to oxalic acid monoacyl chloride (8.72 g), and kept in ice bath for 30 min. Then the temperature rose slowly to 60°C, and a white precipitate precipitated out of the solution. The reaction was continued for 3h, and the solution was filtered, washed with ice ethanol and ether several times, and dried in vacuo to obtain a white solid N-(5-bromo-2-carboxyanilino ) oxalyl ethyl ester 8.77g, yield 69.6%.
[0029] (2) Synthesis of 6-N,N,N-trimethyl-2-aminocaproic acid methyl ester hydrochloride: Reflux 10mmol (2.24g) lamininine hydrochloride in thionyl chloride for 2h, add 50mmol (1.60g) of methanol, continue to reflux for 5h, evaporate the excess solvent, adjust the pH value to n...
Embodiment 2
[0033](1) Synthesis of N-(5-bromo-2-carboxyanilino) oxalyl ethyl ester: Dilute 40mmol (5.49g) ethyl oxalate monoacyl chloride with 10mL of anhydrous tetrahydrofuran (THF) and place in an ice bath Slowly added dropwise to 40 mL of anhydrous THF solution dissolved with 4-bromo-2-aminobenzoic acid equimolar to oxalic acid monoacyl chloride (8.72 g), and kept in ice bath for 30 min. Then the temperature rose slowly to 60°C, and a white precipitate precipitated out of the solution. The reaction was continued for 3h, and the solution was filtered, washed with ice ethanol and ether several times, and dried in vacuo to obtain a white solid N-(5-bromo-2-carboxyanilino ) oxalyl ethyl ester 6.34g, yield 50.3%.
[0034] (2) Synthesis of 6-N,N,N-trimethyl-2-aminocaproic acid methyl ester hydrochloride: Reflux 10mmol (2.24g) lamininine hydrochloride in thionyl chloride for 2h, add 50mmol (1.60g) of methanol, continue to reflux for 5h, evaporate the excess solvent, adjust the pH value to ne...
Embodiment 3
[0037] (1) Synthesis of N-(5-bromo-2-carboxyanilino) oxalyl ethyl ester: Dilute 40mmol (5.49g) ethyl oxalate monoacyl chloride with 10mL of anhydrous tetrahydrofuran (THF) and place in an ice bath Slowly added dropwise to 40 mL of anhydrous THF solution dissolved with 4-bromo-2-aminobenzoic acid equimolar to oxalic acid monoacyl chloride (8.72 g), and kept in ice bath for 30 min. Then the temperature rose slowly to 60°C, and a white precipitate precipitated out of the solution. The reaction was continued for 3h, and the solution was filtered, washed with ice ethanol and ether several times, and dried in vacuo to obtain a white solid N-(5-bromo-2-carboxyanilino ) 8.53g of ethyl oxalate, yield 67.7%.
[0038] (2) Synthesis of 6-N,N,N-trimethyl-2-aminocaproic acid methyl ester hydrochloride: Reflux 10mmol (2.24g) lamininine hydrochloride in thionyl chloride for 2h, add 50mmol (1.60g) of methanol, continue to reflux for 5h, evaporate the excess solvent, adjust the pH value to neu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com