A new 18α-glycyrrhetinic acid derivative and its medical application
A technology of glycyrrhetinic acid and its derivatives, which is applied in the field of medicine and can solve problems such as limitations in clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 8
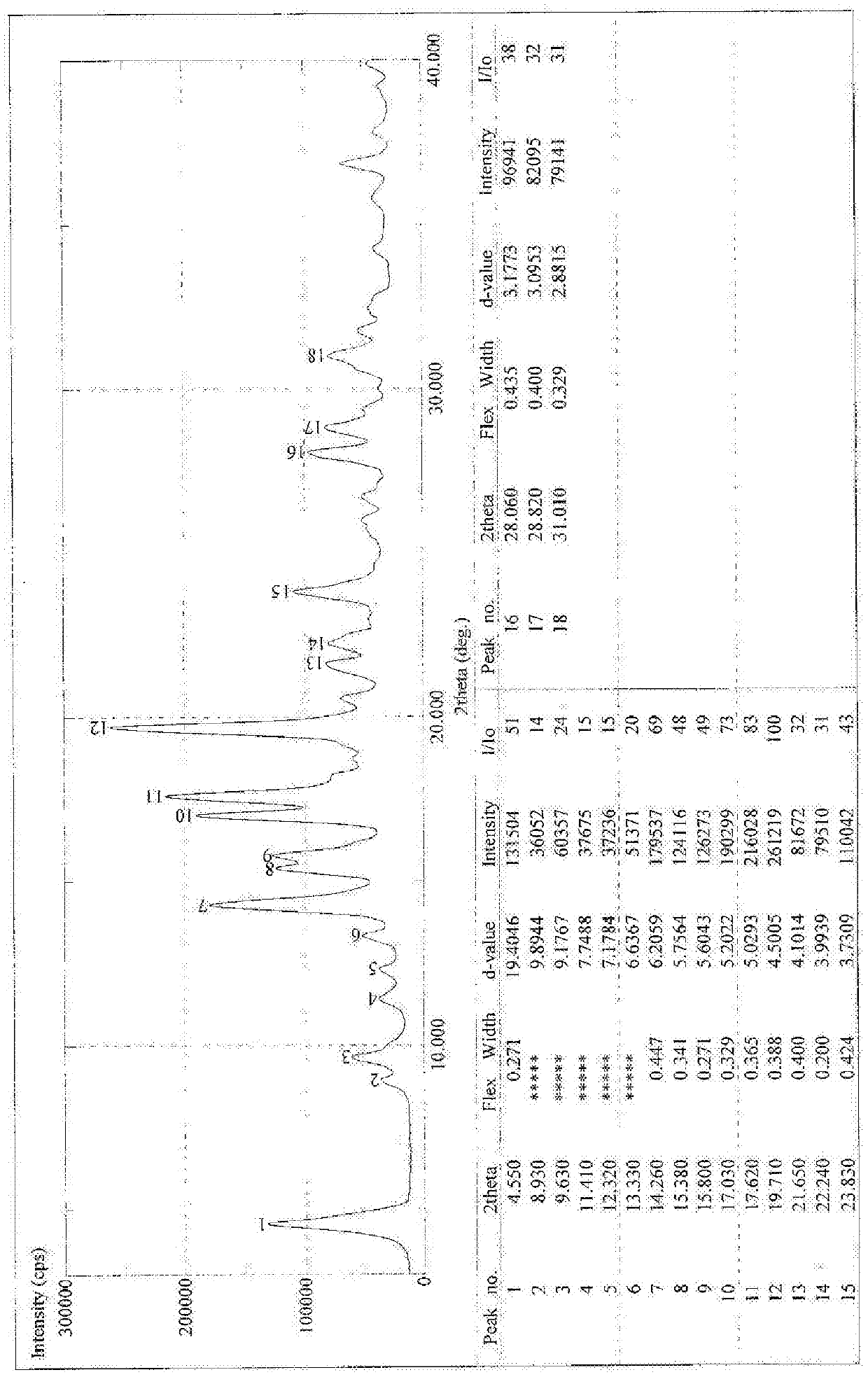
[0044] Example 1 Synthesis and Crystal Form Preparation of 18α-Glycyrrhetinic Acid Choline Salt
[0045] Synthesis of 18α-ethyl glycyrrhetinate, the structure of which is shown in formula (II)
[0046] Add 2kg of 18α-diamine glycyrrhizinate into 11L of absolute ethanol, add dropwise 1L of acetyl chloride, after the dropwise addition is complete, heat and reflux for 2 hours. TLC detects that the reaction is complete. Evaporate to dryness, add 4L of dichloromethane to dissolve, wash with water, take the dichloromethane layer, evaporate to dryness, and refine with ethanol / water, yield: 70%.
[0047] Structural Confirmation:
[0048] (1)MS m / z[M+H] + : 499 quasi-molecular ion peaks and [M++H-H 2 O] + : The fragment ion peak of 481, so the molecular weight of this product is 482.
[0049] (2) NMR:
[0050] Testing unit: China Pharmaceutical University Analysis and Testing Center
[0051] Instrument: BRUKER AV-500 Nuclear Magnetic Resonance Apparatus
[0052] Solvent: CDCl ...
Embodiment 2 8
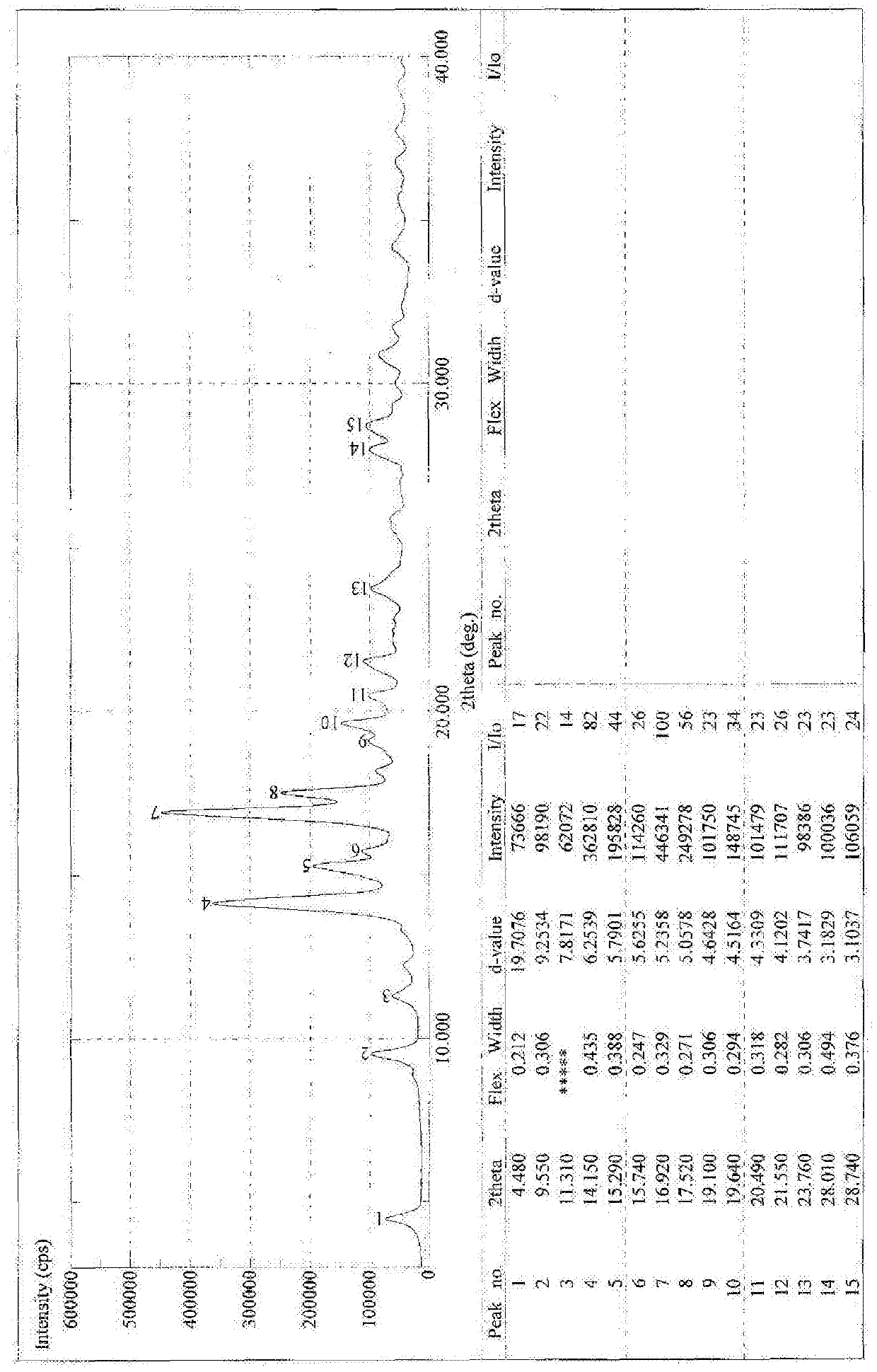
[0069] Example 2 Synthesis of 18α-sodium glycyrrhetinate
[0070] Synthesis of 18α-Ethyl Glycyrrhetinate
[0071] Add 2kg of 18α-diamine glycyrrhizinate into 11L of absolute ethanol, add dropwise 1L of acetyl chloride, after the dropwise addition is complete, heat and reflux for 2 hours. TLC detects that the reaction is complete. Evaporate to dryness, add 4L of dichloromethane to dissolve, wash with water, take the dichloromethane layer, evaporate to dryness, and refine with ethanol / water, yield: 70%.
[0072] Synthesis of 18α-glycyrrhetinic acid
[0073] Take 50g of ethyl 18α-glycyrrhetinate and add it into 450ml of ethanol, add 16g of sodium hydroxide, heat to reflux, and react for about 2 hours. Evaporate to dryness under reduced pressure, add 400ml of water, slowly add hydrochloric acid under stirring to adjust pH = 1-2, a white solid precipitates, and is filtered. Dry at 50°C for 5 hours and then at 80°C to obtain 44.3g. Yield 96.7%.
[0074] Synthesis of 18α-sodium...
Embodiment 3 8
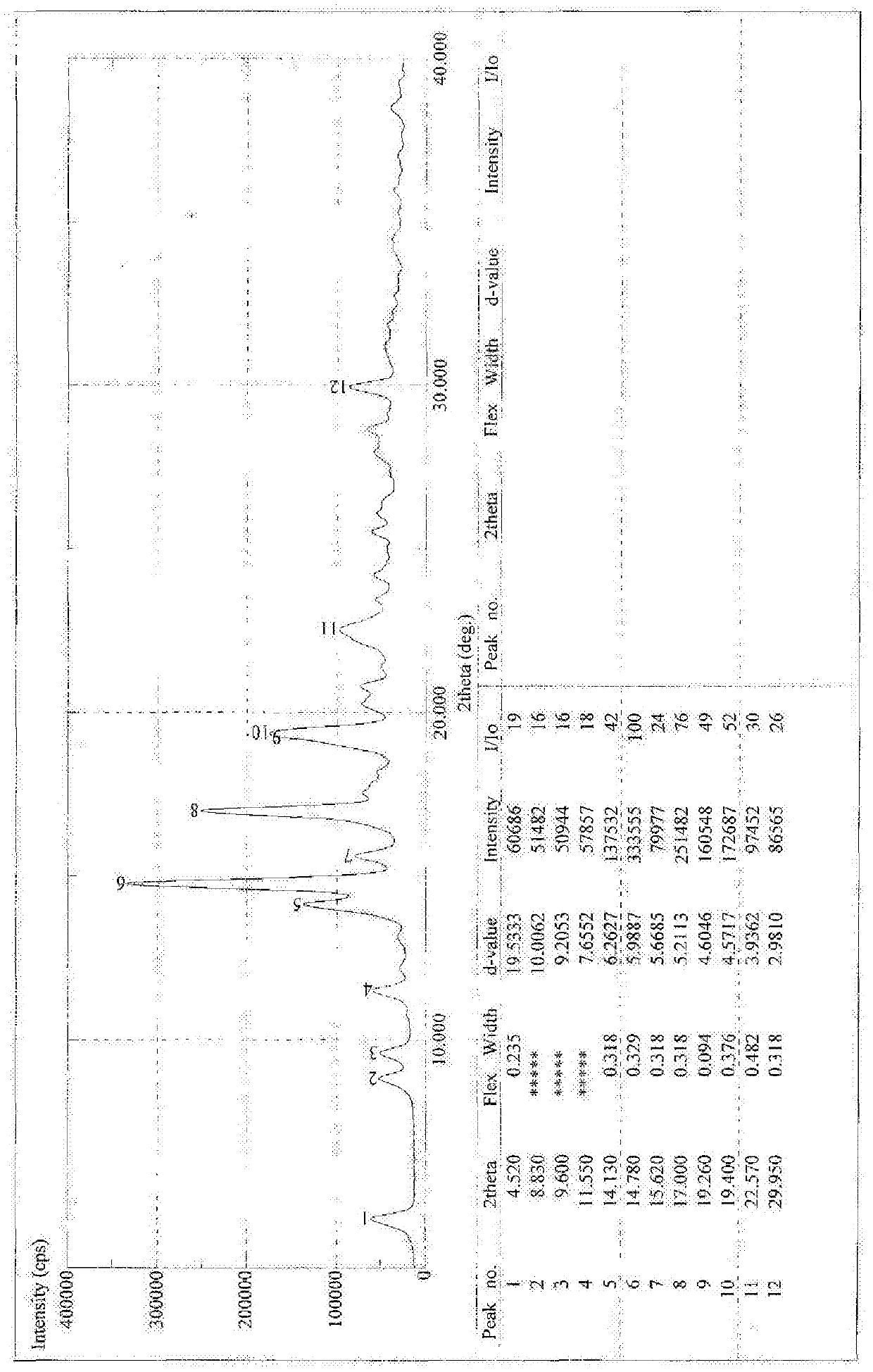
[0076] Example 3 Synthesis of 18α-glycyrrhetinic acid arginine salt
[0077] Synthesis of 18α-Ethyl Glycyrrhetinate
[0078] Add 2kg of 18α-diamine glycyrrhizinate into 11L of absolute ethanol, add dropwise 1L of acetyl chloride, after the dropwise addition is complete, heat and reflux for 2 hours. TLC detects that the reaction is complete. Evaporate to dryness, add 4L of dichloromethane to dissolve, wash with water, take the dichloromethane layer, evaporate to dryness, and refine with ethanol / water, yield: 70%.
[0079] Synthesis of 18α-glycyrrhetinic acid
[0080] Take 50g of ethyl 18α-glycyrrhetinate and add it into 450ml of ethanol, add 16g of sodium hydroxide, heat to reflux, and react for about 2 hours. Evaporate to dryness under reduced pressure, add 400ml of water, slowly add hydrochloric acid under stirring to adjust pH = 1-2, a white solid precipitates, and is filtered. Dry at 50°C for 5 hours and then at 80°C to obtain 44.3g. Yield 96.7%.
[0081] Synthesis of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com