Method for manufacturing drug-containing biodegradable fiber material by electrospinning
A manufacturing method and fibrous technology, applied in the field of locally implanted slow-release agents in the body, and thermoplastic biodegradable resins are used as spinning solutions for electrospinning to prepare the field, which can solve the problems of carrier residues in the body and risks , to achieve excellent local sustained release and improve the effect of QOL
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0098] The present invention can be described in more detail with reference to the following Examples, Comparative Examples and Reference Examples, but is not limited to these Examples.
reference example 1
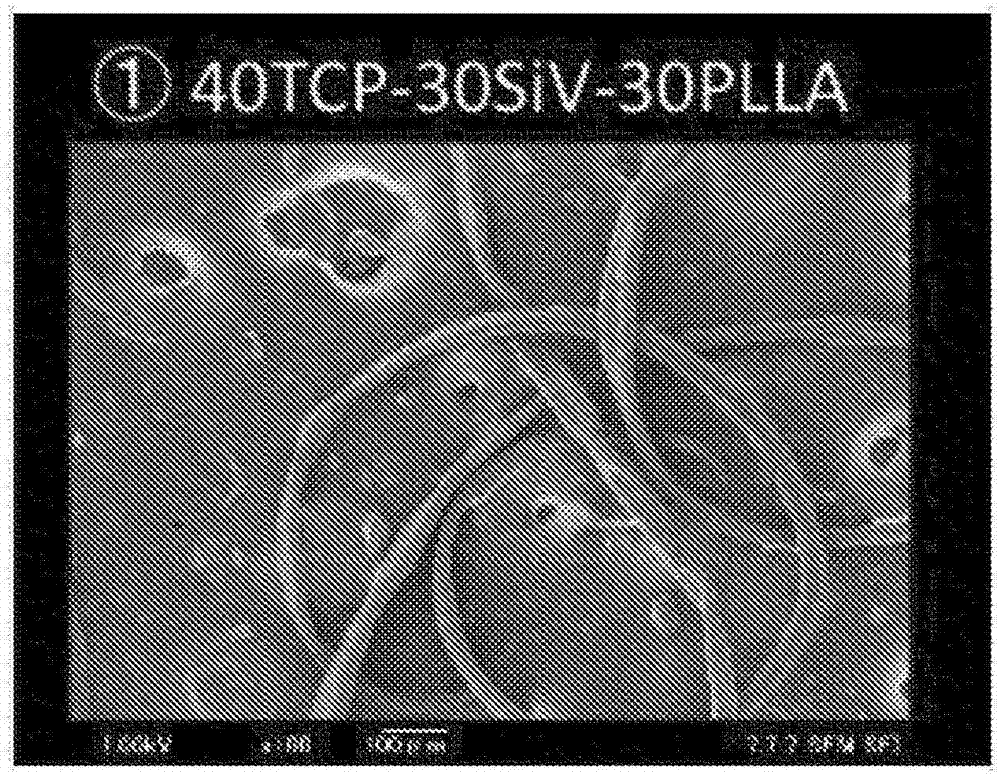
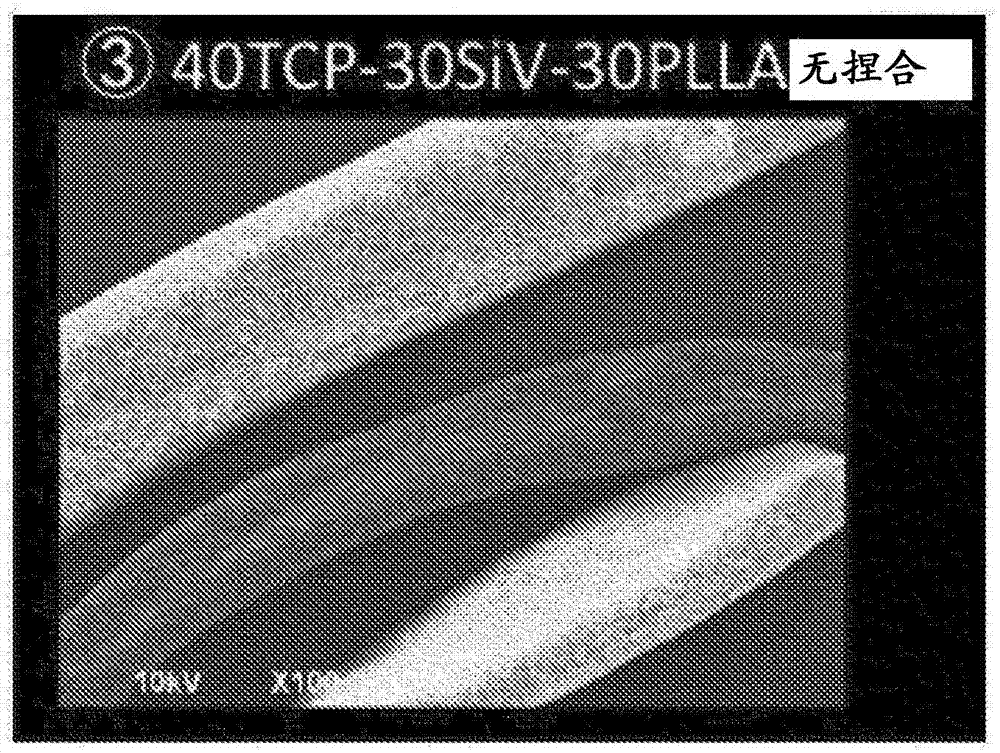
[0099] PLLA, β-tricalcium phosphate and Si-containing moletite phase calcium carbonate (40TCP-30SiV-30PLLA)
[0100] step 1
[0101] Drugs and polylactic acid are mixed and kneaded by a kneader. Preheat the kneader to The set temperature, and then 15g of poly L lactic acid pellets (PURAC PL24, molecular weight: Melting point: ) into the kneader, in Heat mixing and kneading at the set temperature for about 4 minutes. Then, a powder obtained by mixing 20 g of β-tricalcium phosphate powder and 15 g of SiV powder was added to a kneader, and the mixture was further mixed and kneaded at the same set temperature for about 10 minutes.
[0102] When heating at a set temperature of 180° C. to 190° C. of the kneader, mixing and kneading can be performed by applying torque from the kneader in this state. Although the state of the poly-L-lactic acid heated by the kneader is not completely clear, according to the speculation of the present inventors, it is considered that there...
reference example 2
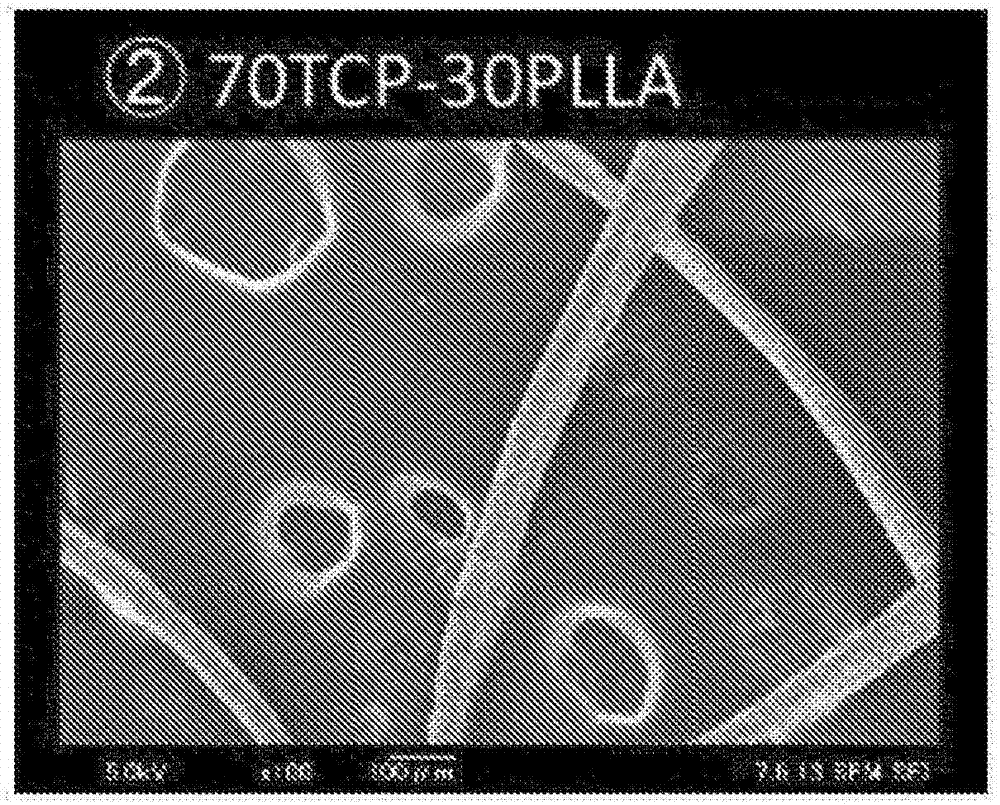
[0115] β-tricalcium phosphate and PLLA (70TCP-30PLLA)
[0116] step 1
[0117] Knead β-tricalcium phosphate and poly-L-lactic acid (PURAC PL24, molecular weight: ).
[0118] Set the kneader set temperature to Preheat for 3 minutes, then add 15g of poly-L lactic acid pellets into the kneader, heat and knead at a set temperature of 180°C to 190°C for about 4 minutes. Then, 35 g of β-tricalcium phosphate powder was added to the kneader, and the two were mixed, and further kneaded at the same set temperature for about 10 minutes.
[0119] The mixture may be kneaded by applying torque from the kneader in this state while heating at a set temperature of 180° C. to 190° C. of the kneader. The state of the poly-L-lactic acid heated by the kneader is not completely clear. According to the estimation of the inventors of the present invention, it is considered that there are portions in a molten state having reached the melting point of poly-L-lactic acid itself and portions in a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com