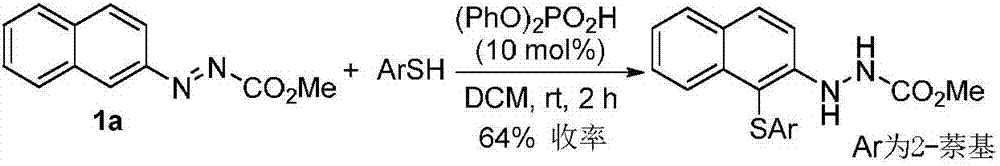
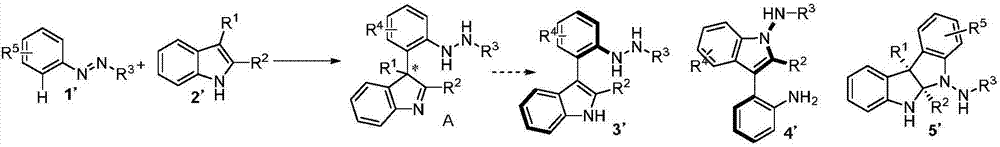
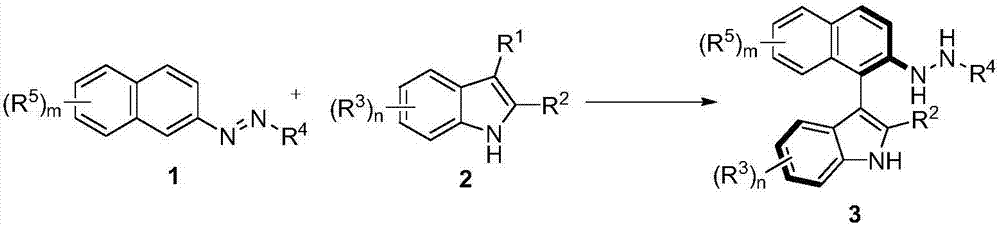
Method for synthetizing axial chirality aryl indole through organocatalysis
A technology of aryl indole and axial chirality, which is applied in the field of organocatalytic synthesis of axial chiral aryl indole, achieving the effects of mild reaction conditions, excellent enantioselectivity, and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of substrate 1
[0057]
[0058] Preparation of aromatic ring-substituted β-naphthylamines 1i-aaa (R 5 =6-Me), 1k-aaa(R 5 =6-Ph), 1l-aaa(R 5 =7-MeO), other β-naphthylamines are commercially available.
[0059] first step:
[0060] According to reference 3, in an ice-water bath, the NaNO 2 (513 mg, 5.76 mmol) of H 2 O (1 mL) solution was slowly added to a suspension of the corresponding amine (4.5 mmol) in hydrochloric acid (5 mL), the resulting solution was stirred in an ice-water bath for 1 h, and SnCl was added slowly 2 ·2H 2 O (3.556 g, 15.76 mol), the resulting suspension was stirred in an ice-water bath for 3.5 hours and then filtered. Sequentially at 0 °C with H 2 O (4×8mL), H at room temperature 2 O(1×8mL), Et 2 The solid was washed with O / n-hexane (1:1, 2 x 4 mL) and dried to give the desired product.
[0061]
[0062] Following the general procedure, 1i-aa was obtained in 96% yield.
[0063] 1 H NMR (400MHz, DMSO-d 6 )δ10.46(s,3H),...
Embodiment 2
[0103] Synthesis of substrate 2
[0104]
[0105] 2a, 2i are commercially available, other indoles were prepared according to refs 6-10.
[0106] General method for the synthesis of 2-(tert-butyl)-1H-indole
[0107]
[0108] The corresponding phenylhydrazine hydrochloride (0.72 g, 5 mmol) and pinacolone (5 mL, 40 mmol), ZnCl 2 (2.72 g, 20 mmol) were mixed and heated with an oil bath at 190°C, the reaction mixture was kept at this temperature for 20 minutes, and after completion of the reaction monitored by TLC, the reaction mixture was cooled to room temperature, diluted with water, and extracted with ethyl acetate (2 × 50 mL), the combined organic phases were washed with water and washed with Na 2 SO 4 After drying and evaporation of the solvent, the residue was subjected to silica gel column chromatography using PE / EA as elution solvent to give the product.
[0109] 2b, yellow solid, 77% yield, 0.73 g.
[0110] 1 H NMR (500MHz, CDCl 3 )δ7.91(s,1H),7.23–7.15(m,2H...
Embodiment 3
[0119] In order to verify the feasibility of the reaction, as shown in the following formula, the azobenzene derivatives 1a and 2-tert-butyl-indole 2a were used as reactants, and 10 mol% phosphoric acid CP1 was used as a catalyst to react in DCM at room temperature. The reaction proceeded smoothly, and the axially chiral aryl indole 3a was obtained in 76% yield and 87% ee. It can be seen that through the nucleophilic attack of azobenzene derivatives by indole, it is feasible to asymmetrically construct axial chiral aryl indole through organocatalysis. Next, the catalysts with different axial chiral skeletons and substituents were screened. The aromatic ring skeleton and 3,3'-substituents of the catalysts have important effects on the enantioselectivity. Among them, catalyst CP4 had the best results in terms of enantioselectivity (92% ee) and yield (99%).
[0120]
[0121] Reaction conditions: 1a (0.10 mmol, 1.0 equiv), 2a (0.12 mmol, 1.2 equiv) and CP (10 mol%) were reacte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com