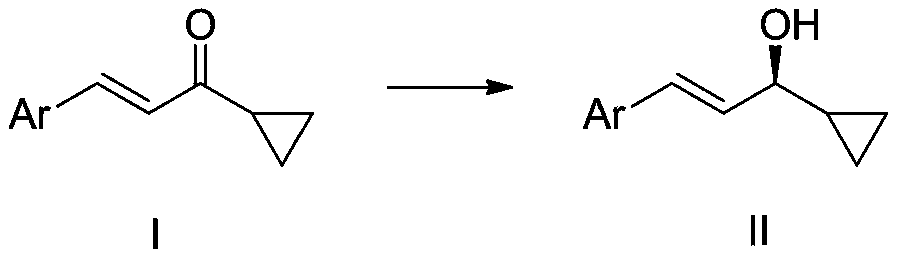
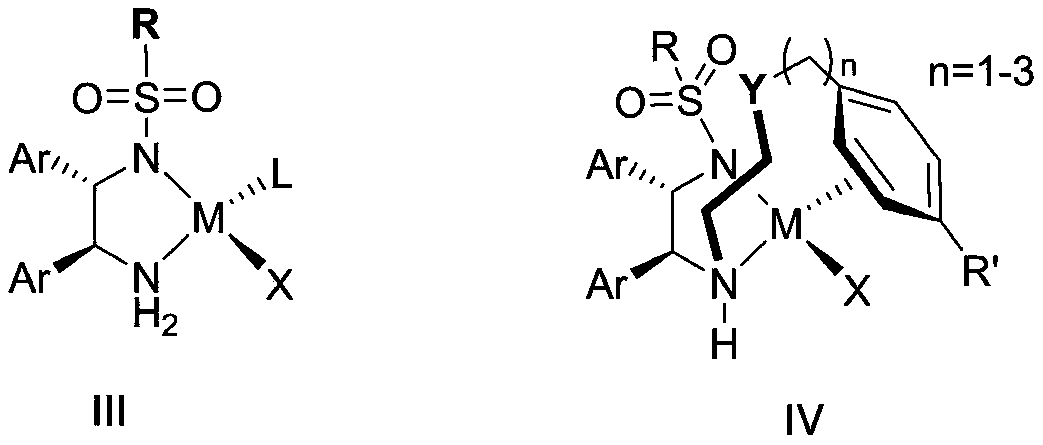
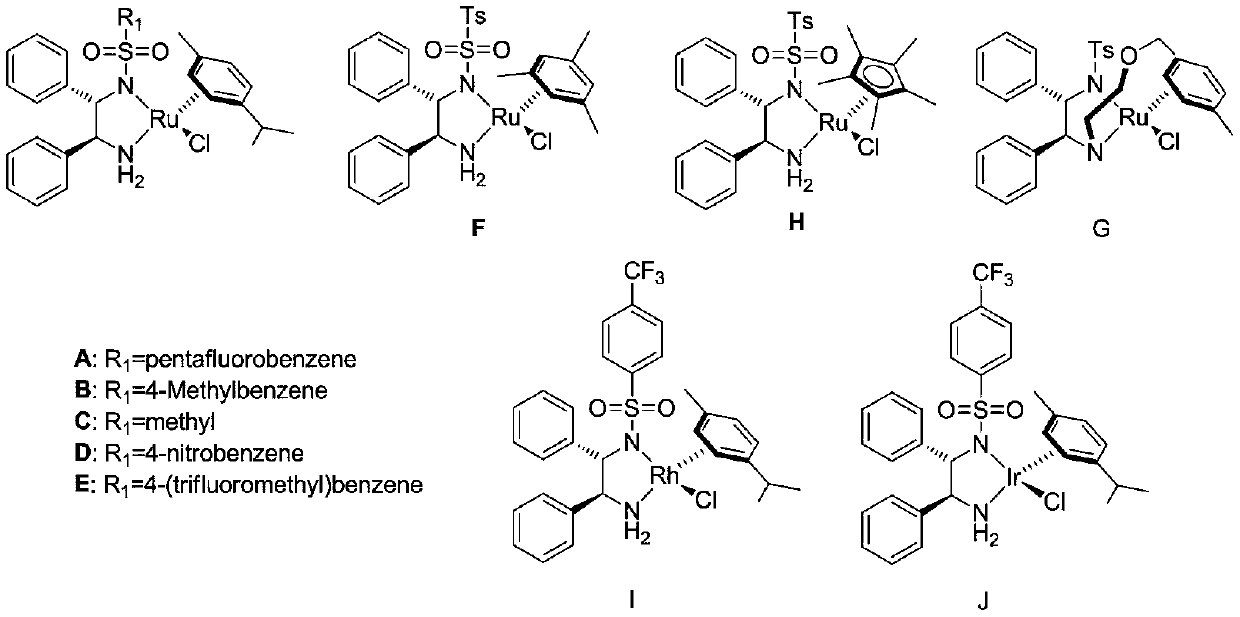
A kind of cyclopropyl substituted allyl alcohol and its asymmetric synthesis method
A technology of allyl alcohol and cyclopropyl, which is applied in the field of asymmetric catalysis to achieve the effect of cheap price, stable ligand and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Catalyst A-J, p-(E)-styryl cyclopropanemethanol asymmetric synthesis
[0029]
[0030] Add 0.01 mmol of catalysts numbered A-J to 10 ml of Schlenk test tubes, add 0.2 mmol (E)-styryl cyclopropanone, add hydrogen source, different solvents, seal the test tubes, and replace the gas with nitrogen three times , 50°C for 24 hours. After the reaction, wash with water, extract the aqueous phase with ethyl acetate 3 times, combine the organic phases and concentrate to dryness, use catalyst E, formic acid: triethylamine (5:2, V / V) when the yield and enantiomeric excess value The highest, the isolated yield is 90% (petroleum ether: ethyl acetate = 3:1), the ee value of the enantiomeric excess of 81% of the product (S,E)-styryl cyclopropanol was determined by HPLC, and the results are shown in Table 1 shown. HPLC separation conditions: chiral column Daicel OD-H column, mobile phase: n-hexane / isopropanol = 90:10 (volume ratio), flow rate: 1.0 ml / min, wavelength: ...
Embodiment 2
[0034] Example 2: Asymmetric Synthesis of (S, E)-(4-Methylstyryl) Cyclopropanol
[0035]
[0036] Catalyst E (0.01mmol, 3.26mg) was added to a 10ml Schlenk test tube, and 0.2mmol (E)-(4-methylstyryl) cyclopropanone, formic acid: triethylamine (5:2, v / v) 1 mL, seal the test tube, replace the gas with nitrogen three times, and react at 50°C for 24 hours. After the reaction was finished, the reaction was washed with water, the aqueous phase was extracted 3 times with ethyl acetate, combined and concentrated to dryness, the isolated yield: 93% (petroleum ether: ethyl acetate = 3:1), and the product (S, E)-( 4-Methylstyryl)cyclopropanemethanol has an enantiomeric excess of 73% ee. HPLC separation conditions: chiral column Daicel OD-H column, mobile phase: n-hexane / isopropanol=95:5 (volume ratio), flow rate: 1.0 ml / min, wavelength: 254 nm, column temperature: 30 degrees Celsius, t1 = 12.28 minutes, t2 = 14.00 minutes; 1H NMR (400MHz, CDCl 3 ): δ=7.33(t, J=7.2Hz, 2H), 7.16(t, ...
Embodiment 3
[0037] Example 3: Asymmetric Synthesis of (S, E)-4-trifluoromethylstyryl) cyclopropanemethanol
[0038]
[0039] Catalyst E (0.01mmol, 3.26mg) is added in the Schlenk test tube of 10 milliliters, adds 0.2mmol0.2mmol (E)-(4-trifluoromethyl styryl) cyclopropanone, formic acid: triethylamine ( 5:2, v / v) 1mL, seal the test tube, replace the gas with nitrogen three times, and react at 50°C for 24 hours. Washed with water after the reaction, the aqueous phase was extracted 3 times with ethyl acetate, combined and concentrated to dryness, the isolated yield: 95% (petroleum ether: ethyl acetate=3:1), HPLC assay product (S, E)-( 4-Trifluoromethylstyryl)cyclopropanol has an enantiomeric excess of 82% ee. HPLC separation conditions: chiral column Daicel OD-H column, mobile phase: n-hexane / isopropanol=99:1 (volume ratio), flow rate: 1.0 ml / min, wavelength: 254 nm, column temperature: 30 degrees Celsius, t1=30.51 minutes, t2=32.84 minutes; 1 H NMR (400MHz, CDCl 3 ):δ=7.60(d, J=8.4Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com