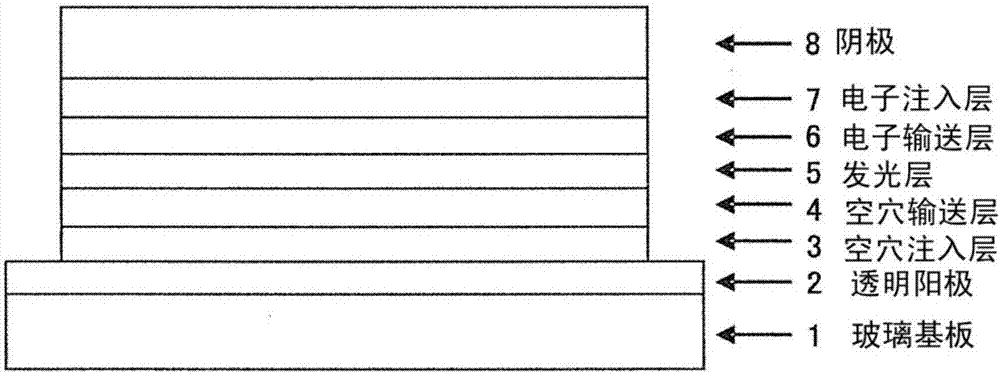
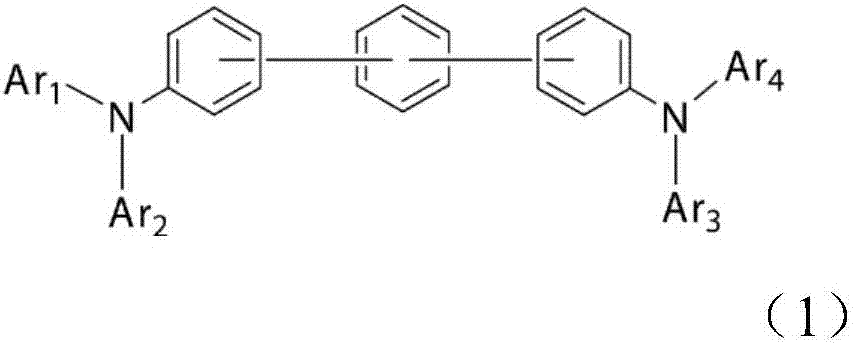
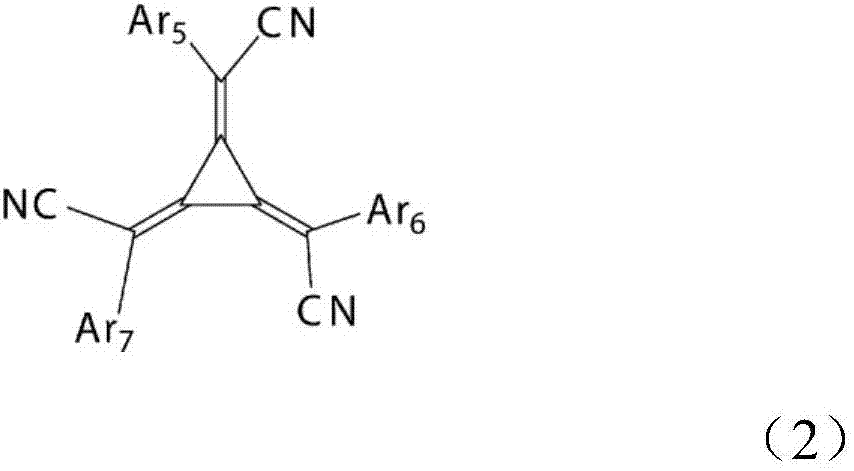
Organic electroluminescent element
An electroluminescent device and a luminescent technology, which are applied in the direction of organic semiconductor devices, electric solid devices, electrical components, etc., can solve problems such as insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241]
[0242]Add (biphenyl-4-yl)-aniline 39.5g, 4,4"-diiodo-1,1'; 4',1"-terphenyl 32.4g, copper powder 0.42g, potassium carbonate 27.8 g to the reaction vessel g, 1.69 g of 3,5-di-tert-butylsalicylic acid, 2.09 g of sodium bisulfite, 32 ml of dodecylbenzene, and 50 ml of toluene, and heated to 210°C while distilling off the toluene. After stirring for 30 hours, it cooled, and 50 ml of toluene and 100 ml of methanol were added. The precipitated solid was collected by filtration, washed with 500 ml of a mixed solution of methanol / water (5 / 1, v / v), then 350 ml of 1,2-dichlorobenzene was added and heated, and insoluble matter was removed by filtration. After natural cooling, 400 ml of methanol was added, and the precipitated crude product was collected by filtration, and reflux washing was performed with 500 ml of methanol to obtain 4,4"-bis{(biphenyl-4-yl)-phenylamino}-1 ,1'; 4',1"-terphenyl (compound 1-1) gray powder 45.8g (91% yield).
[0243]
[0244] The structure of...
Embodiment 2
[0248]
[0249] 16.7 g of (biphenyl-4-yl)-4-toluidine, 12.9 g of 4',1"-terphenyl, 0.17 g of copper powder, 11.2 g of potassium carbonate, 0.71 g of 3,5-di-tert-butylsalicylic acid, 0.89 g of sodium bisulfite, 20 ml of dodecylbenzene, and 20 ml of toluene were heated to 210°C while distilling off the toluene, and stirred for 28 hours. After cooling, 150 ml of toluene was added, and insoluble matter was removed by filtration. Add 100ml of methanol, collect the precipitated crude product by filtration, and repeat recrystallization three times with a mixed solvent of toluene / methanol to obtain 4,4"-bis{(biphenyl-4-yl)-4-toluene 12.3 g (yield: 61%) of yellow-white powder of 1-amino}-1,1'; 4',1"-terphenyl (compound 1-10).
[0250]
[0251] For the obtained yellow-white powder, NMR was used to identify the structure.
[0252] use 1 H-NMR (CDCl 3 ) detected the following 44 hydrogen signals.
[0253] δ (ppm) = 7.68-7.62 (4H), 7.61-7.41 (16H), 7.38-7.08 (18H), 2.38 (6H).
Embodiment 3
[0255]
[0256] Add (biphenyl-4-yl)-(phenyl-d 5 )amine 25.3g, 4,4"-diiodo-1,1'; 4',1"-terphenyl 20.3g, copper powder 0.30g, potassium carbonate 17.5g, 3,5-di-tert-butyl salicylic acid 1.05g, 1.31g of sodium bisulfite, 20ml of dodecylbenzene, and 30ml of toluene were heated to 210°C while distilling off the toluene. After stirring for 23 hours, it cooled, and 30 ml of toluene and 60 ml of methanol were added. The precipitated solid was collected by filtration, washed with 180 ml of a mixed solution of methanol / water (1 / 5, v / v), and then washed with 90 ml of methanol. 210 ml of 1, 2- dichlorobenzene was added to the obtained gray powder, it heated, and the insoluble matter was removed by filtration. After natural cooling, 210ml of methanol was added, and the precipitated crude product was collected by filtration, and 210ml of methanol was used for reflux washing to obtain 4,4"-bis{(biphenyl-4-yl)-(phenyl-d 5 )amino}-1,1'; 29.3g (yield: 96%) of gray powder of 4',1"-terphenyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com