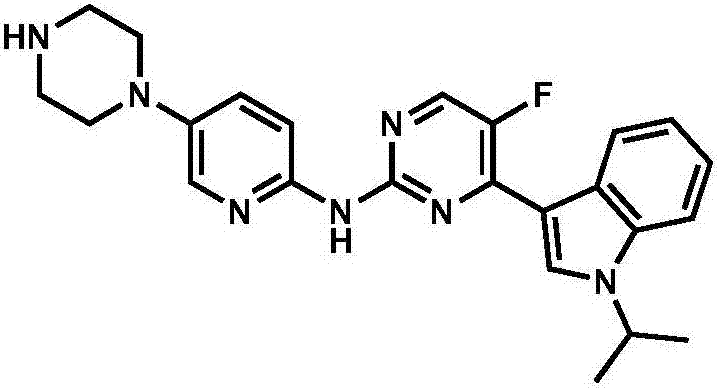
Application of pyrilamine compound as cycle protein dependency kinase 4/6 inhibitor
A technology of pyrimidine amines and compounds, applied in the field of medicinal chemistry, can solve problems such as low activity, large side effects, and low therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: 5-fluoro-4-(1-isopropyl-1H-indol-3-yl)-N-(5-(piperazin-1-yl)pyridin-2-yl)pyrimidine-2- Synthesis of Amine (Compound (1))
[0059]
[0060] Reaction conditions: i) indole, MeMgBr, 1,2-dichloroethane, 0°C → room temperature; ii) NaH, THF, 0 → 35°C, 48h; iii) Pd 2 (dba) 3 , Xantphos, DIPEA, dioxane, 100°C, 48h; iv) hydrogen chloride gas, ethyl acetate, overnight at room temperature.
[0061] The first step: the synthesis of 3-(2-chloro-5-fluoropyrimidin-4-yl)-1H-indole (intermediate 1)
[0062] 1.17g indole (10.0mmoL) was dissolved in 15mL of 1,2-dichloroethane, 7.5ml of methylmagnesium bromide (15mmoL, 2M THF solution) was added, the temperature was lowered to 0°C, and 1.66g of 2 , 4-dichloro-5-fluoropyrimidine (10.0mmoL) dissolved in 15mL of 1,2-dichloroethane, kept at 0°C for 1 hour, returned to room temperature for 1 hour, added 30ml of water under continuous stirring , separate out a large amount of solids, filter, use water, 10% citric acid and ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com