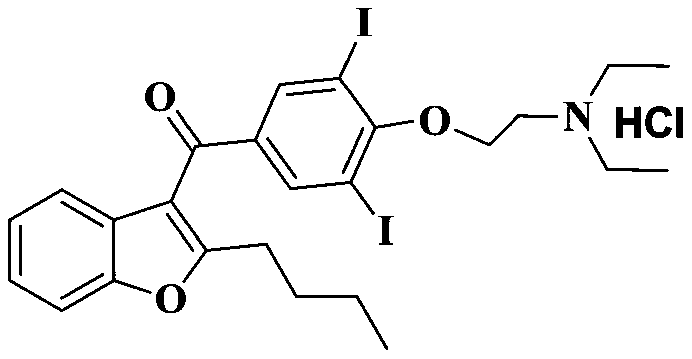
A kind of preparation method of amiodarone hydrochloride
A technology of amiodarone hydrochloride and compounds, which is applied in the field of medicine, can solve the problems of low yield and purity, and achieve the effects of high purity, simple process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The synthesis of embodiment 1 amiodarone hydrochloride
[0050] Synthesis of compound shown in A, formula 3
[0051]
[0052] Add 276.7g (2.0mol) of anhydrous potassium carbonate, 97.6g (0.8mol) of 2-hydroxybenzaldehyde, 100g of N,N-dimethylformamide, and 300g of toluene into a 2L three-necked flask, stir mechanically, and set the temperature at 60-70°C 176.0g (0.84mol) alkyl 2-bromohexanoate was added dropwise at a temperature of 80-100°C, stirred and reacted for 2 hours, 320g of water was added, the water layer was separated, and the organic layer was washed with 320g of water Once, the solvent was evaporated at 0.09MPa / 80°C to obtain 190.2g of the compound represented by formula 3, with a GC purity of 98.8% and a yield of 95.0% (calculated as 2-hydroxybenzaldehyde).
[0053] Synthesis of compound shown in B, formula 4
[0054]
[0055] 200.4g (0.8mol) of the compound shown in formula 3 was added into 2L methanol solution dissolved with 93.4g (0.88mol) of trim...
Embodiment 2
[0079] The synthesis of embodiment 2 amiodarone hydrochloride
[0080] Synthesis of compound shown in A, formula 3
[0081]
[0082] Add 332.0g (2.4mol) of anhydrous potassium carbonate, 97.6g (0.8mol) of 2-hydroxybenzaldehyde, 130g of N,N-dimethylformamide, 600g of toluene into a 2L three-necked flask, stir mechanically, and set the temperature at 60-70°C 176.0g (0.84mol) alkyl 2-bromohexanoate was added dropwise at a temperature of 80-100°C, stirred and reacted for 2 hours, 400g of water was added, the water layer was separated, and the organic layer was washed with 400g of water Once, the solvent was evaporated at 0.09MPa / 80 to obtain 197.2g of the compound shown in formula 3, with a GC purity of 98.6% and a yield of 98.5% (calculated as 2-hydroxybenzaldehyde).
[0083] B, the synthesis of formula 4 compounds
[0084]
[0085] 200.4g (0.8mol) of the compound shown in formula 3 was added into 2L methanol solution dissolved with 127.2g (1.2mol) of trimethyl orthoforma...
Embodiment 3
[0109] The synthesis of embodiment 3 amiodarone hydrochloride
[0110] Synthesis of compound shown in A, formula 3
[0111]
[0112] Add 387.3g (2.8mol) of anhydrous potassium carbonate, 97.6g (0.8mol) of 2-hydroxybenzaldehyde, 140g of N,N-dimethylformamide, 600g of toluene into a 2L three-necked flask, stir mechanically, and set the temperature at 60-70°C 176.0g (0.84mol) alkyl 2-bromohexanoate was added dropwise at a temperature of 80-100°C, stirred and reacted for 2 hours, 500g of water was added, the water layer was separated, and the organic layer was washed with 500g of water Once, the solvent was evaporated at 0.09MPa / 80 to obtain 198.2g of the intermediate of formula 3 with a GC purity of 98.9% and a yield of 99.0% (calculated as 2-hydroxybenzaldehyde).
[0113] Synthesis of compound shown in B, formula 4
[0114]
[0115] 200.4g (0.8mol) of the compound shown in formula 3 was added into 2L methanol solution dissolved with 169.8g (1.6mol) of trimethyl orthoform...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com