Preparation method for 3-sulfo-indoles compound
A technology for thioindole and compounds, which is applied in the field of preparation of 3-thioindole compounds, can solve problems such as complex process, lower performance of 3-thioindole compounds and product yield, and achieve substrate Wide adaptability, good industrial application prospects, and environmentally friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The invention provides a kind of preparation method of 3-thioindole compound, comprises the following steps:
[0053] Perform photocatalytic substitution reaction of thiophenolic compound and indole compound under the action of organic solvent and photocatalyst to obtain 3-thioindole compound;
[0054] The thiophenolic compound has the structure shown in formula I:
[0055]
[0056] The indole compound has a structure shown in formula II:
[0057]
[0058] The 3-thioindole compound has the structure shown in formula III:
[0059]
[0060] R in the formula I 1 is methoxy-substituted phenyl, alkyl-substituted phenyl or halogen-substituted phenyl;
[0061] R in the formula II 2 is hydrogen, alkyl, halogen, methoxy, nitro, nitrile, hydroxymethyl, aminomethyl or trifluoromethyl;
[0062] R 3 is hydrogen, alkyl, aminomethyl, aminoethyl, formyl or N,N-dimethyl;
[0063] R 4 is hydrogen, alkyl, aminomethyl, hydroxymethyl, phenyl or tolyl;
[0064]The photocata...
Embodiment 1
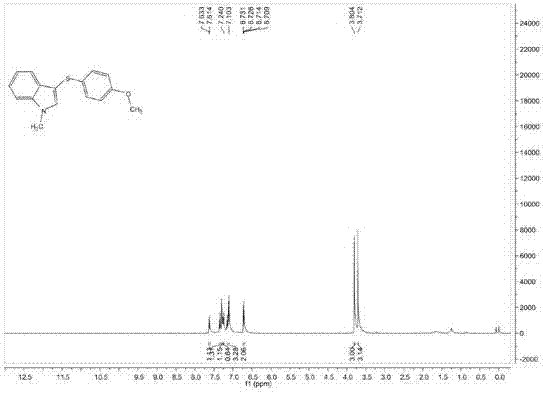
[0092] Add 0.1mmol 1-methylindole, 0.2mmol p-methoxythiophenol, 0.02mmol Rose Bengal, 0.1ml dichloromethane into the reaction flask, and stir the reaction at 22°C under the irradiation of 400nm light source for 20 Hours, after the reaction was completed, it was separated and purified by column chromatography. The volume ratio of petroleum ether and ethyl acetate in the column chromatography eluent was 24:1, and the purified target product was obtained with a yield of 64%. The structural characterization data of the resulting product are as follows:
[0093] 1 H NMR (400MHz, CDCl 3 ): δ=7.62(d, J=8.0Hz, 1H), δ=7.35(d, J=8.0Hz, 1H), δ=7.29(d, J=8.0Hz, 1H), δ=7.24(d, J=8.0Hz, 1H), δ=7.17-7.10(m, 3H), δ=6.71-6.71(m, 2H), δ=3.81(s, 3H), δ=3.71(s, 3H);
[0094] 13 C NMR (100MHz, CDCl 3 )δ=157.7, 137.5, 134.5, 128.4, 119.7, 114.5, 109.7, 102.3, 55.3, 33.1;
[0095] MS (EI, 70eV): m / z (%) = 269 (M+), 254, 237, 222, 210, 162, 135, 120, 102 (100).
[0096] The structural characte...
Embodiment 2
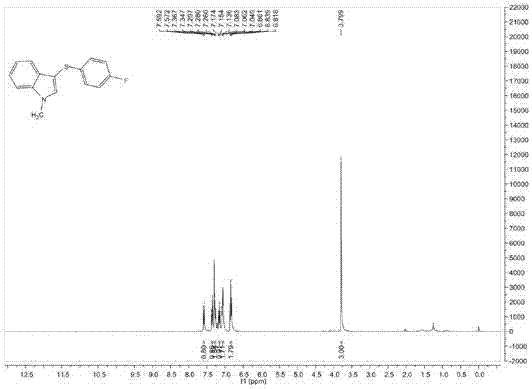
[0100] Add 0.2mmol of 1-methylindole, 0.3mmol of p-fluorothiophenol, 0.002mmol of red Y, 0.2ml of dichloromethane into the reaction bottle, under the irradiation of 350nm light source, stir and react at 26°C for 6 hours, the reaction After completion, separation and purification by column chromatography, the volume ratio of petroleum ether and ethyl acetate in the eluent of column chromatography was 8:1, and the purified target product was obtained with a yield of 30%.
[0101] The structural characterization data of the resulting product are as follows:
[0102] 1 H NMR (400MHz, CDCl 3 ): δ=7.58(d, J=8Hz, 1H), δ=7.36(d, J=8Hz, 1H), δ=7.30-7.26(m, 2H), δ=7.17-7.14(m, 1H), δ=7.08-7.05(m,2H), δ=6.86-6.82(m,2H), δ=3.80(s,3H);
[0103] 13 C NMR (100MHz, CDCl 3 )δ=162.1, 159.7, 137.6, 134.9, 134.5, 129.6, 127.8, 127.7, 122.7, 120.6, 119.6, 115.8, 115.6, 109.8, 101.4, 33.1;
[0104] MS (EI, 70eV): m / z (%) = 257 (M+), 242, 225, 183, 162, 121 (100).
[0105] According to the ab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com