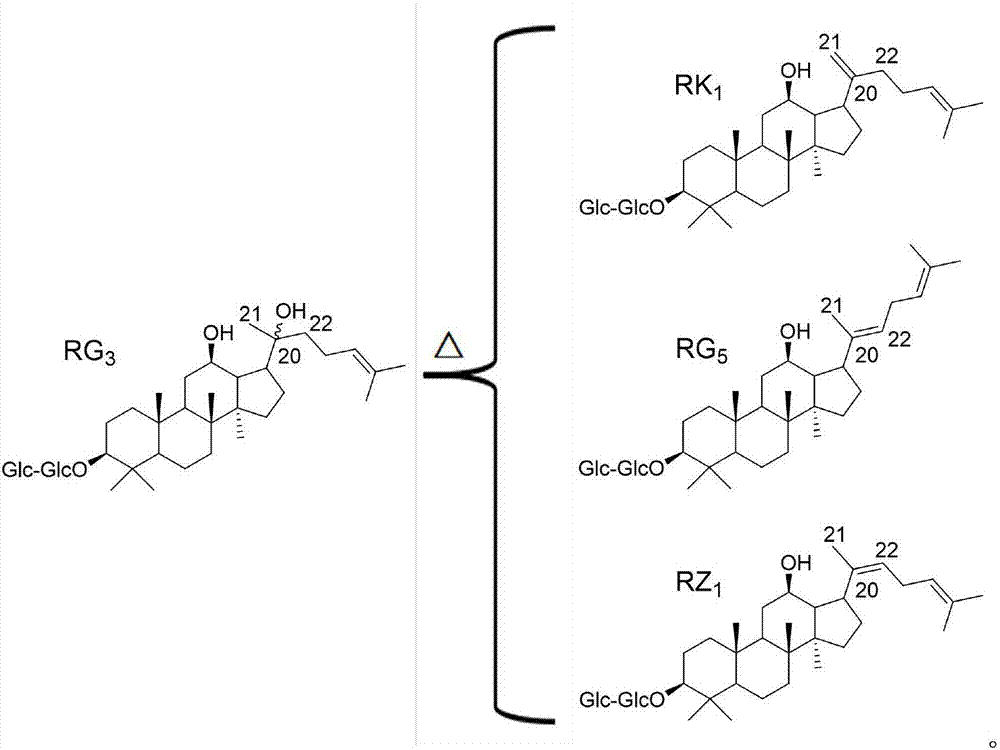
Ginsenoside RZ1 solid dispersion and preparation method thereof
A technology of solid dispersion and ginsenosides, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems of poor water solubility and low bioavailability of ginsenosides, and achieve improved Solubility, stability improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of Ginsenoside RZ by Supercritical Antisolvent Method 1 Solid dispersion
[0028] The granulation equipment is the Helix supercritical crystallization granulation system produced by Applied Separations, USA.
[0029] Ginsenoside RZ 1 Self-made, the purity is greater than 98%.
[0030] Preparation process:
[0031] Start the device, and after the temperature of the crystallization tank reaches 55°C, the CO 2 Compressed by a high-pressure pump and preheated by a preheater, it is passed into the crystallization kettle from the top of the kettle to 14MPa. After the temperature and pressure in the kettle are stabilized, open the CO2 at the bottom of the kettle. 2 Outlet valve, and adjust the fine-tuning valve to the predetermined volume flow rate of 3L / min.
[0032] Ginsenoside RZ 1 Dissolve PVP-K30 in a mixed solvent of acetone and dichloromethane (volume ratio of acetone and dichloromethane 1:1) in a mass ratio of 1:3 to prepare ginsenoside R...
Embodiment 2
[0033] Embodiment 2 Solvent stirring method prepares ginsenoside RZ 1 Solid dispersion
[0034] A certain amount of ginsenoside RZ 1 Dissolve in absolute ethanol with povidone K-30 at a mass ratio of 1:3 to configure ginsenoside RZ 1 The solution with a mass concentration of 15mg / mL was magnetically stirred at room temperature for 3h (100r / min), and the solvent was removed by rotary evaporation at 50°C under reduced pressure, dried in vacuum for 24h, crushed through a No. 5 sieve, and stored in a desiccator for future use.
Embodiment 3
[0035] Embodiment 3 Comparative Examples
[0036] Replace the solid carrier with β-cyclodextrin, and the others are the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com