Ophthalmic preparation of sirolimus or derivative thereof
The technology of an ophthalmic preparation, sirolimus, is applied in the field of drug ophthalmic drug delivery preparations to achieve the effects of easy filling, simple preparation method and accurate dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
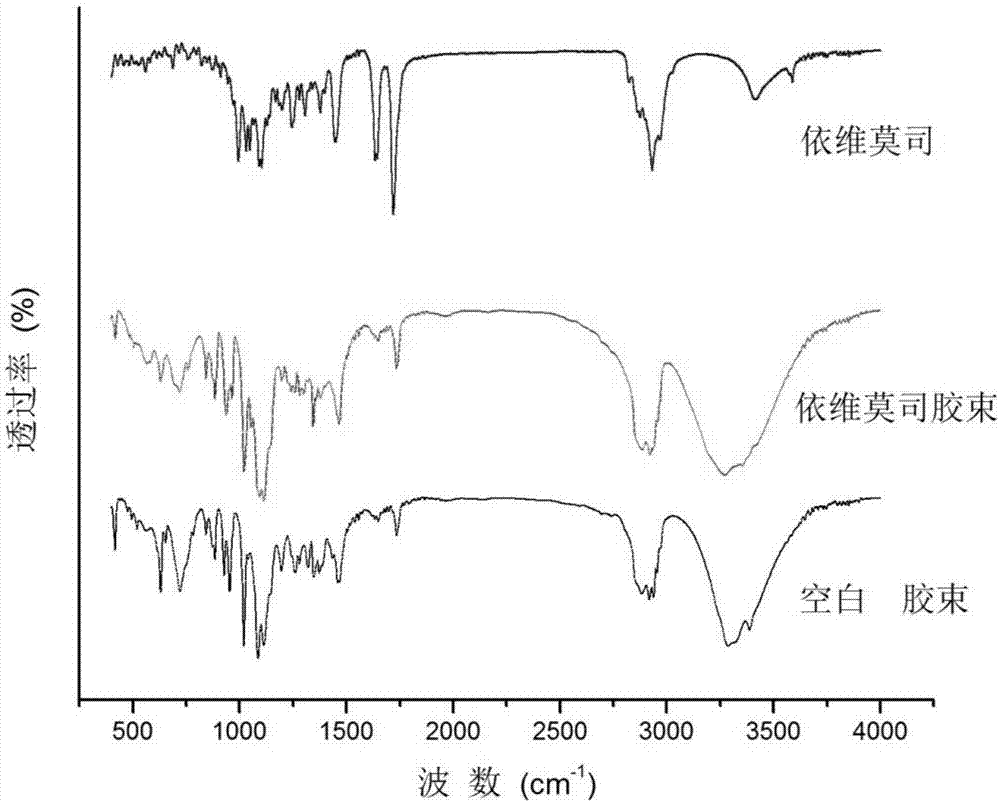
[0056] Everolimus micelles
[0057] Composed of the following raw materials in weight percent:
[0058]
[0059] Preparation method: Prepared by an improved film hydration method, the specific method is as follows: First, dissolve everolimus and polyethylene glycol 40 stearate in ethanol according to the above ratio, and the polymer is in the flask after rotary evaporation to remove the solvent ethanol Form a thin film on the surface, add hydration medium containing osmotic pressure regulator mannitol, antibacterial agent benzalkonium chloride and polysorbate 80, oscillate ultrasonically, driven by hydration force, water molecules penetrate into the polymer through the gap film, forming micelles.
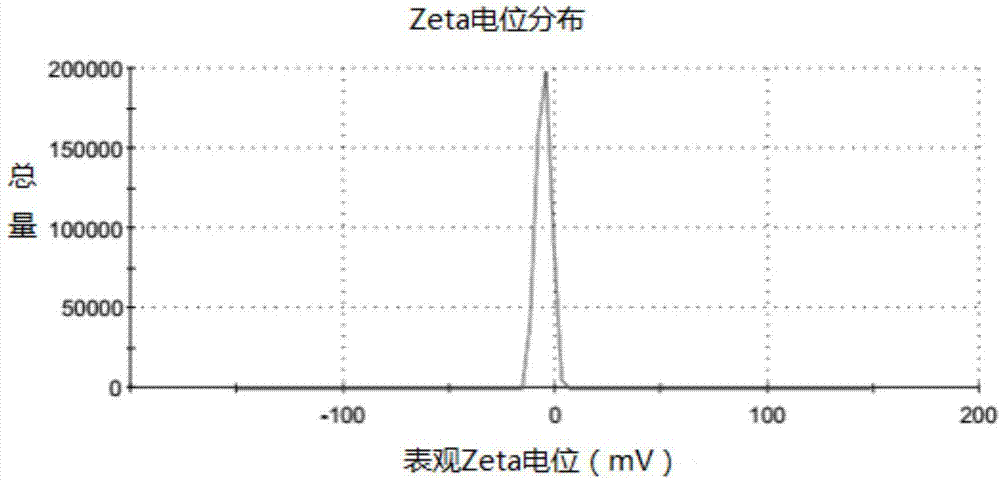
[0060] After testing, the pH value of the obtained everolimus micelles was 7.1; the osmotic pressure was 293 mOsmol / kg. The drug loading is 2%, and the encapsulation efficiency is 93.0%.
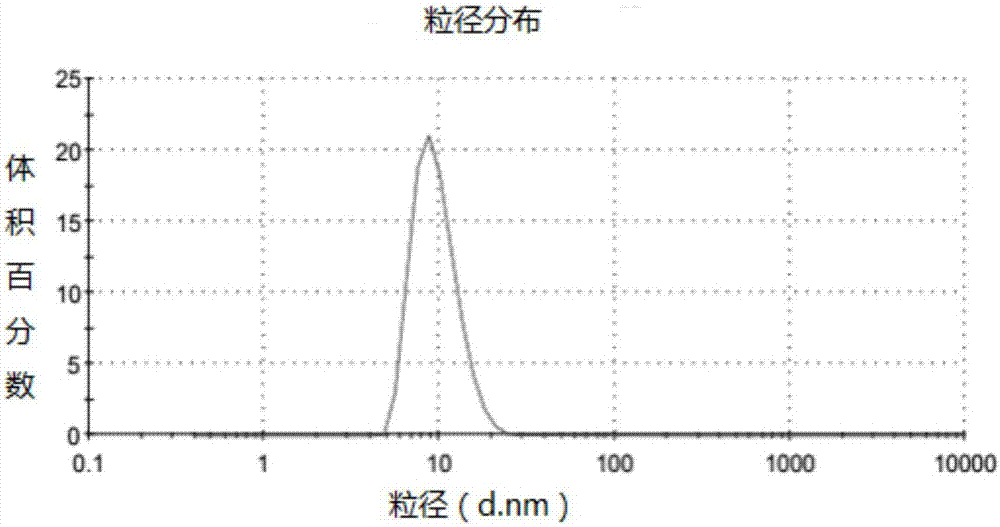
[0061] Get the obtained everolimus micelles, add deionized water to disperse, and measur...
Embodiment 2
[0065] everolimus suspension
[0066] Composed of the following raw materials in weight percent:
[0067]
[0068] Preparation method: first prepare a polyvinyl alcohol (PVA) solution, weigh 1.4g of low-viscosity PVA, add purified water to about 100mL, stir and dissolve in a water bath at 80-85°C, and let it sit for a certain period of time to swell to form a PVA solution. Then weigh 100 mg of everolimus, add 2 g of propylene glycol to dissolve everolimus. Add the above-mentioned everolimus solution into the PVA solution dropwise, and keep stirring. Add 0.02g of benzalkonium chloride, and finally quantify the suspension to 100mL, and sonicate for 10min.
[0069] After testing, the pH value of the obtained everolimus suspension was 5.7; the osmotic pressure was 286 mOsmol / kg.
[0070] Take an appropriate amount of the resulting everolimus suspension, add deionized water to disperse, and measure the particle size with a Malvern particle size potentiometer. The results are ...
Embodiment 3
[0086] Everolimus micellar in situ gel
[0087] Composed of the following raw materials in weight percent:
[0088]
[0089] Preparation method: preparation by improved film hydration method. The specific method is as follows: First, dissolve everolimus and polyethylene glycol 40 stearate in a certain amount of ethanol according to the above ratio, and the polymer forms a film on the inner surface of the flask after the solvent is removed by rotary evaporation. Separately take hypromellose and gellan gum, add water and stir to dissolve in a water bath at 80-85°C, add osmotic pressure regulator mannitol, bacteriostatic agent benzalkonium bromide and polysorbate 80 to 100mL to form a hydration medium . Add the hydration medium into the above flask and shake it. Driven by the hydration force, water molecules penetrate into the polymer film through the gap to form a micelle-in-situ gel composite carrier.
[0090] After testing, the pH value of the obtained everolimus micella...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com