Vascular endothelial growth factor fusion protein
A growth factor, fusion protein technology, applied in the direction of anti-growth factor immunoglobulin, growth factor/growth regulator, fusion polypeptide, etc., can solve the problem of abnormal VEGF angiogenesis, etc. Excellent inhibitory activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0054] Hereinafter, the present invention will be described in more detail with reference to Examples. It should be understood that these examples are not to be construed as limiting the invention in any way.
Embodiment 1
[0056] Example 1: Production of VEGFR fusion protein KP-VR2
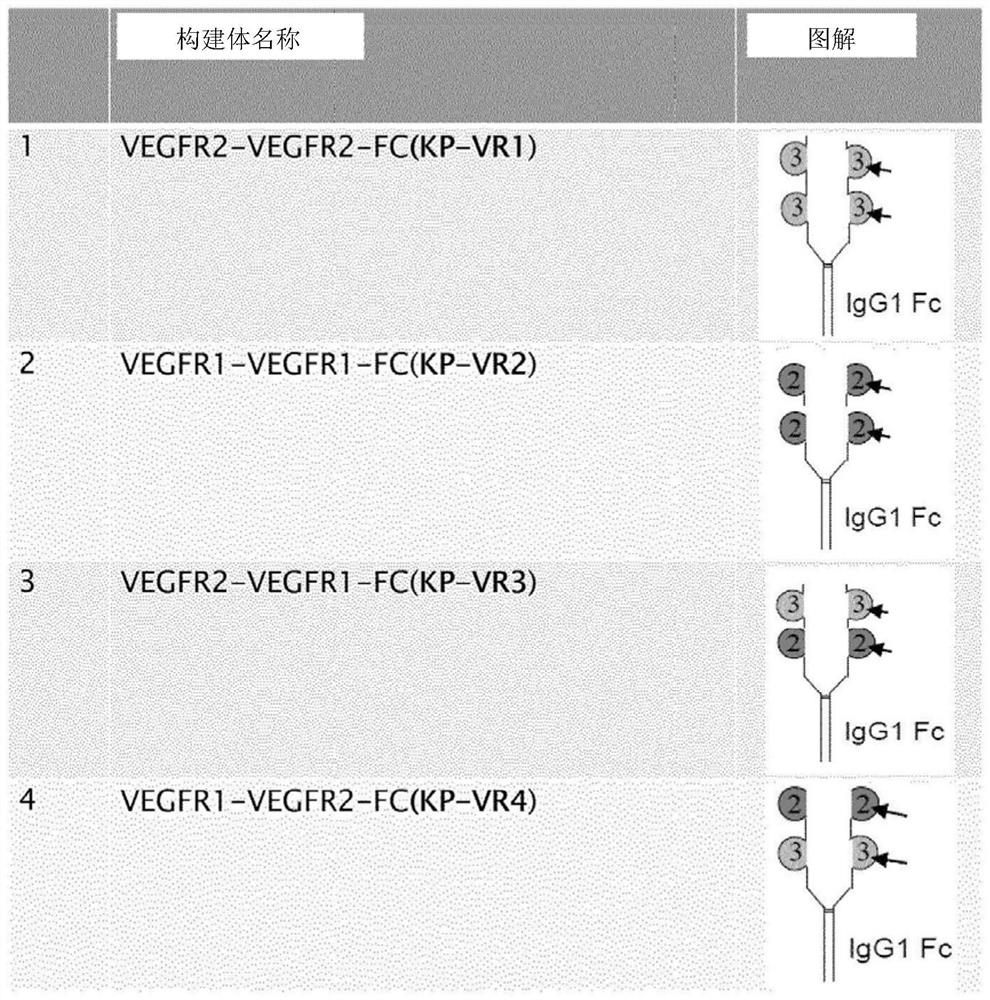
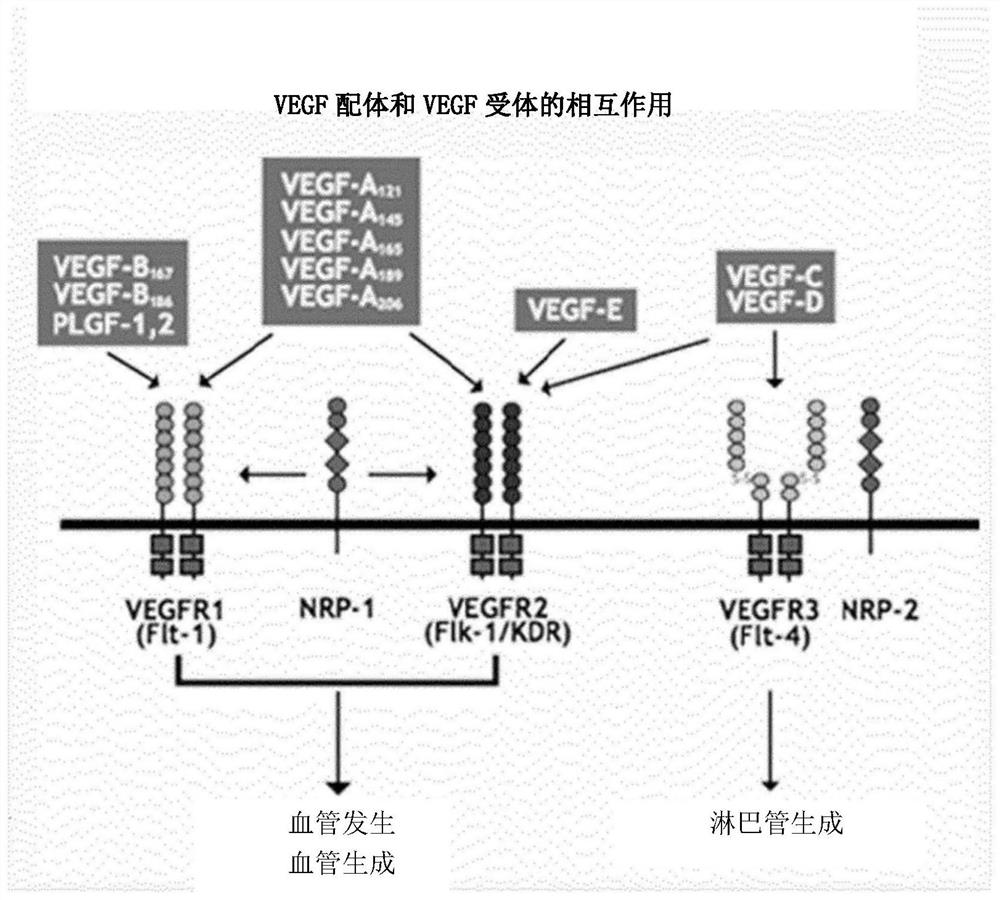
[0057] human VEGFR2 domain 3 (KP-VR1) fused to a human IgG1 Fc domain; human VEGFR1 domain 2 (SEQ ID NO: 1) (KP-VR2) fused to a human IgG1 Fc domain (SEQ ID NO: 2); Human VEGFR2 domain 3 (SEQ ID NO:3) and VEGFR1 domain 2 (KP-VR3) fused to a human IgG1 Fc domain; human VEGFR1 domain 2 and VEGFR2 domain 3 fused to a human IgG1 Fc domain (KP-VR3) VR4, aflibercept) were cloned into pCHO 1.0 vector (Invitrogen) respectively.
[0058] CHO-S cells (Invitrogen) were transfected with KP-VR1, KP-VR2, KP-VR3 and KP-VR4 constructs, respectively, and transfected cells were selected by using methotrexate and puromycin. KP-VR2 and KP-VR4 were purified by protein A-agarose affinity chromatography (VEGF-Trap; Aflibercept; Eylea). Purified proteins were quantified by HPLC analysis followed by SDS-PAGE and Western blotting sequentially. figure 1 Each construct is shown. image 3 Indicates the results of western blotting. like ...
Embodiment 2
[0059] Example 2: Determination of the binding affinity of KP-VR2, aflibercept and bevacizumab of the present invention to VEGF-A
[0060] In this experiment, the binding affinities of KP-VR2, aflibercept and bevacizumab of the present invention to VEGF-A were compared. The ELISA assay was performed according to the BEVACIZUMAB Summary Validation Report (February 28, 2014). Coat 96-well plates (petri dishes) with 50ng / mL VEGFA165(I) or VEGFA121(III) (R&D Systems), and then KP-VR2, KP-VR4 (aflibercept) or Avastin (bevacizumab) Antibody) was added to the wells in increasing amounts from 0.0122 to 1,000 ng / mL. Next, the plate was washed and reacted with a peroxidase-conjugated anti-human Fc antibody. Then, 3,3,5,5-tetramethylbenzidine (TMB) solution (Roche) was added, and then the absorbance was measured at 450 nm using an ELISA reader (spectrophotometer, Biorad). Figure 5 (I and III) show the results.
[0061] In addition, 96-well plates were coated with 2 nM of KP-VR2 or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com