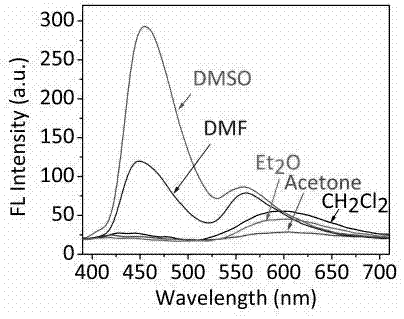
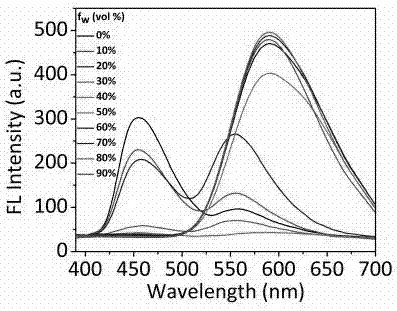
Compound based on aggregation-induced emission and excited state proton transfer and preparation method and application thereof
A technology of aggregation-induced luminescence and proton transfer, which is used in material excitation analysis, chemical instruments and methods, luminescent materials, etc., and can solve the problems of short emission wavelength, low fluorescence quantum yield, and low sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of compound I of the present invention
[0033] (1)
[0034]
[0035] Tetraphenylethylene hydroxyl derivatives, compound 1 (2.0 g, 5.8 mmol), MgCl 2 (1.7 g, 17.8 mmol) and paraformaldehyde (1.2 g, 40 mmol) were dissolved in anhydrous THF (40 mL), then triethylamine (3.2 mL, 23 mmol) was added, and the reaction mixture was refluxed overnight at 75°C . For post-treatment, the reaction was first quenched with 1 mol / L hydrochloric acid solution, then extracted with dichloromethane, and finally compound 2 was obtained as a yellow solid by column chromatography.
[0036] 1 H NMR (400 MHz, CDCl 3 ): δ 10.94 (s, 1H), 9.58 (s, 1H), 7.19 (s, 2H),7.18 – 7.09 (m, 9H), 7.05 (s, 6H), 6.73 (d, J = 8.2 Hz, 1H); 13 C NMR (101 MHz, CDCl 3 ): δ 196.90, 160.44, 143.65, 143.63, 143.33, 141.76, 140.44, 139.14,136.74, 135.95, 131.65, 131.57, 131.53, 128.36, 128.24, 128.05, 127.15,127.10, 126.94, 120.43, 117.26; HRMS (ESI): m / z [M-H] - calcd for C 27 h 20 o 2 ,375.1...
Embodiment 2
[0042] Using compound I as the parent compound, the fluorescent probe L obtained after modification is applied to biothiol imaging, as follows:
[0043]
[0044] Under anhydrous and oxygen-free conditions, triethylamine (21 μL, 0.15 mmol) was added to a solution of compound Ⅰ (50 mg, 0.11 mmol) in dichloromethane, stirred at -78°C for 10 min, and then 2,4- Dinitrobenzenesulfonyl chloride (38 mg, 0.15 mmol) was stirred at room temperature for 2 h, and finally purified to obtain probe L as a yellow solid.
[0045] 1 H NMR (400 MHz, CDCl 3 ): δ 8.20 (s, 1H), 7.81 (dd, J = 20.2, 7.6 Hz, 3H),7.72 (d, J = 7.5 Hz, 1H), 7.45 (d, J = 14.8 Hz, 2H), 7.34 (d, J = 6.9 Hz,1H), 7.21 (d, J = 17.1 Hz, 5H), 7.16 – 7.06 (m, 10H), 7.03 (s, 2H); 13 C NMR (101 MHz, CDCl 3 ): δ 162.47, 153.18, 150.00, 148.36, 144.86, 144.68, 143.65,143.20, 142.71, 138.66, 135.91, 135.10, 134.69, 134.28, 132.99, 131.68,131.65, 131.46, 128.47, 128.39, 128.12, 127.46, 127.38, 127.29 , 127.00, 126.70, 1...
Embodiment 3
[0047] We can derive a class of compounds with both AIE and ESIPT mechanisms and more applications, as follows:
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com